Amino acid analysis by liquid chromatography and optical detection requires additional sample preparation, because most amino acids lack a chromophore and cannot be detected. Thus, after hydrolysis, the next step in an amino acid analysis is derivatization. This section describes the preparation of protein or peptide hydrolysates for derivatization using Waters AccQ•Tag chemistry.
To properly and reliably derivatize a hydrolyzed sample, the following must be considered:
The following discussions are abbreviated out of necessity. For additional information and guidance on the AccQ•Tag derivatization chemistry, please visit www.waters.com/AAA.
Centrifugation may be necessary, if large amounts of particulates or floating lipids are present. Centrifugation makes it is easier to withdraw an aliquot of clear hydrolysate for derivatization.
For large volume samples such as feed analysis hydrolysates, filtration of the particulates may be enough, noting that sample recoveries may be affected by choice of filter material. This factor needs to be considered and addressed in order to obtain unbiased results. Contact the filter manufacturer for details regarding filter performance in this application.
The AccQ•Tag Method is a pre-column derivatization technique for amino acid analysis of hydrolyzed peptide and proteins. The AccQ•Tag Method accomplishes the following:
The 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC) reagent reacts with both primary and secondary amines. Excess AQC reagent reacts with water to form 6-aminoquinoline (AMQ). AMQ reacts slowly with excess AQC reagent to form a bis urea. These side products do not interfere with the separation, identification, and quantitation of the amino acids contained in the peptide or protein hydrolysates. The derivatives are stable for days, permitting batch processing or repeat analysis, if desired.
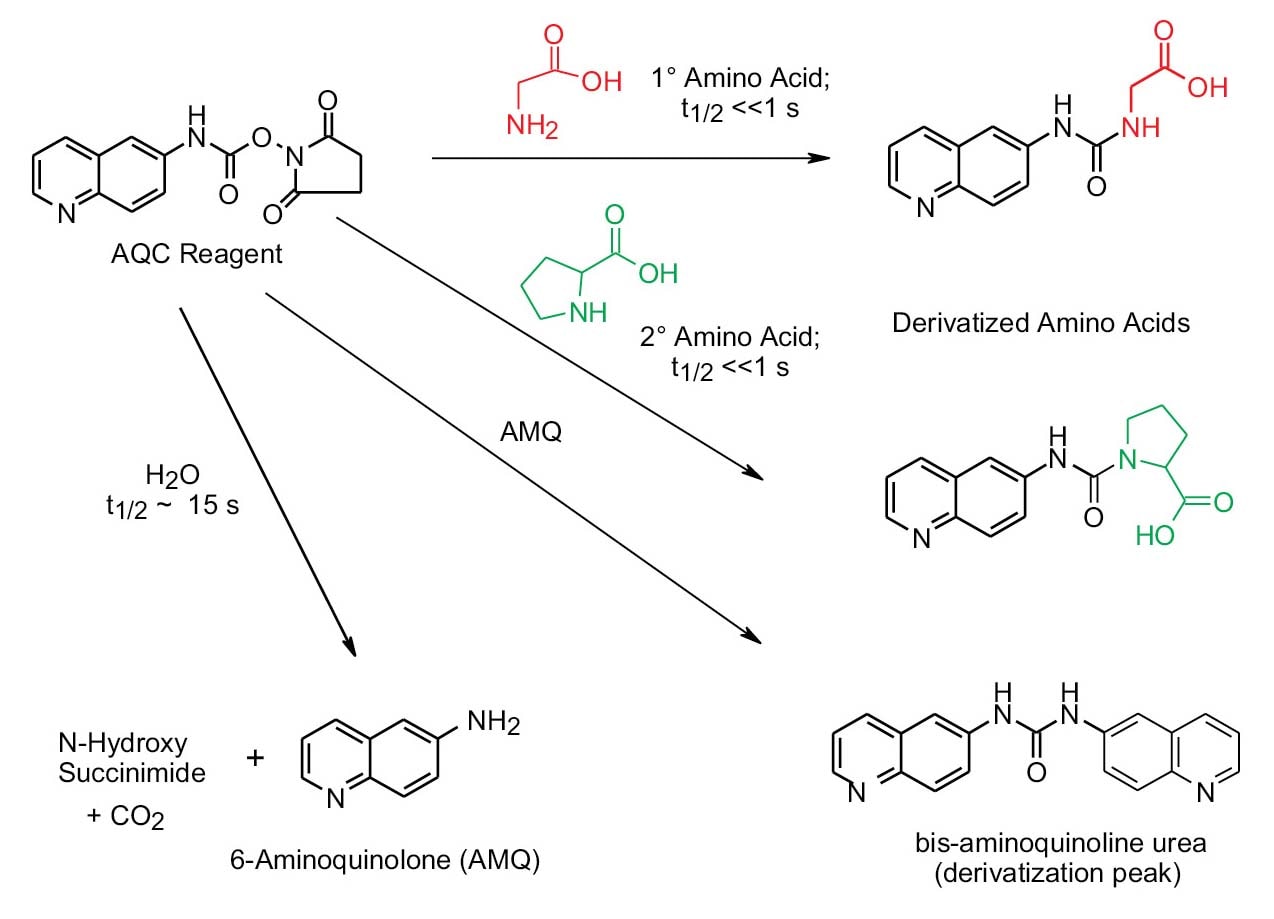 Figure 10. Schematic of AccQ•Tag reaction from derivitization of primary and secondary amino acids.
Figure 10. Schematic of AccQ•Tag reaction from derivitization of primary and secondary amino acids.
Over many years, extensive studies have been performed to ensure the accuracy of the AccQ•Tag derivation reaction. The chemical reaction itself requires both a molar excess of the derivatization reagent and basic pH (8–10) for complete derivatization of all the amino acids. Strategies have been developed to address these critical factors.
In the reaction itself, the sample is typically diluted 10x, and 1 µL out of a 100 µL total derivatization volume is injected onto the column. Because not all amino acids are present in the same amount in a derivatization, the amount of sample must be sufficient for the least abundant amino acid to impact the limit of detection or quantitation. Derivatization of at least 1 pmol of the least abundant amino acid, in a maximum injection volume of 1 µL, is recommended to obtain accurate quantitation. To determine the amount of sample needed, perform the following calculation:
For a sample concentration of 1.0 mg/mL protein:
Estimate the amount of sample needed for the least abundant amino acid. In this example, we assume 0.03 mg/mL is needed to deliver 1 pmol on column of this amino acid.
Convert mg to moles (average molecular weight of an amino acid residue in a protein is 110).
![]()
This is the estimated concentration of the least abundant amino acid in this sample.
![]()
This gives a 27-fold dilution (10 ÷ 0.37 = 27).
The hydrolysate requires a 27-fold dilution prior to derivatization. For example: 5 µL of the sample hydrolysate can be diluted by 135 µL of 0.1 N HCl to yield this target value.
Finally, a 10 µL aliquot of the above dilution can be transferred to the derivatization vial.
To ensure complete AccQ•Tag reagent derivatization of the amino acids contained in the hydrolysate, the sample must be buffered in an approximate pH range of 8.2 to 10.1. If the acid hydrolysate is not properly neutralized, and if the pH falls below 8.2, derivatization will be incomplete. The effect of pH varies for each amino acid. Note that not all of the amino acids are affected equally. Acidic amino acids, such as glutamic acid and alanine, are impacted more than serine or phenylalanine. pH is a critical factor in obtaining accurate quantitation of all the amino acids in the original sample.
WARNING: If the sample turns bright yellow upon addition of the derivatization reagent, the pH of the sample is too low. Neutralize with NaOH.
To determine the amount of NaOH needed for neutralization, perform the following calculation.
For the above sample of protein at 1.0 mg/mL in 6 N HCl, which needs to be diluted 27x to ensure that there is enough excess derivatization reagent in the sample, the following calculations apply.
Convert the acid concentration of the sample from molar to µmoles:
![]()
Determine the final concentration of acid in the diluted sample:
Reminder: 5 µL of the sample hydrolysate can be diluted by 135 µL of 0.1 N HCl to yield this target value.
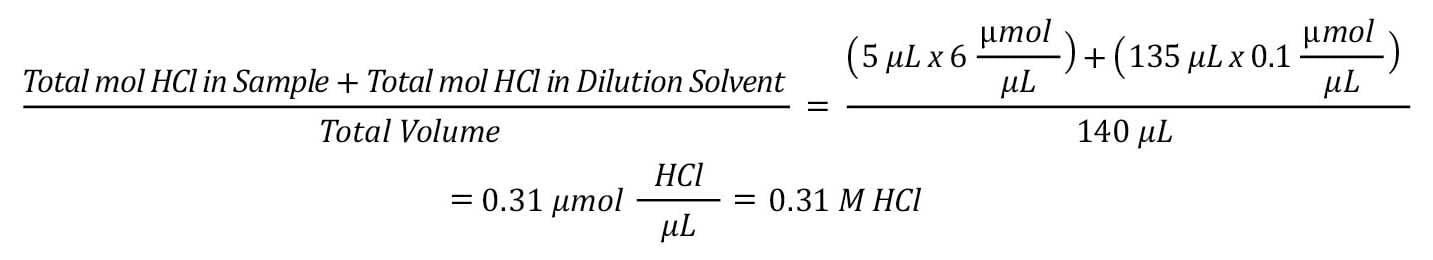
The total amount of NaOH needed per derivatization is 0.31 M.
Since the total borate buffer added to derivatization is 70 µL, there are two methods for neutralization:
Add 10 µL of 0.31 M NaOH and 60 µL of buffer for each derivatization.
In a separate vial, mix 600 µL of borate buffer and 100 µL of 0.31 M NaOH. Mix. Add 70 µL of this mixture + 10 µL sample + 20 µL AccQ•Tag derivatization reagent for each sample.
For complete derivatization of all the amino acids, 4–6x molar excess of the AccQ•Tag derivatization reagent is needed in the reaction. If there is insufficient reagent, some relatively sensitive amino acids will not be completely derivatized. The rates of derivatization for each amino acid vary based on the chemical properties of the amino acids; for example, the recovery of alanine can be significantly affected by insufficient molar excess of AccQ•Tag, while phenylalanine is more immune to these effects.
To determine the amount of sample to add to the reagent vial, we need to know the amount of reagent in each vial. The standard AccQ•Tag reagent vial contains 3–4 mg of reagent, which is approximately 10–14 µmoles of reagent. Because the reagent is dissolved in 1 mL of acetonitrile, and 20 µL is used for each 100 µL derivatization reaction, each reaction vessel contains 210–280 nmoles of derivatization reagent.
Because each reaction vial contains 210–280 nmol of reagent, and we need 4–6x molar excess for each sample, there should be no less than 40–140 nmoles of total amines in each reaction.
For a protein sample, use the weight of sample and the average weight of an amino acid to estimate the required excess.
For example, with a 1-mg/ml protein concentration, and an average molecular weight of 110, the amount of protein in the sample is determined as follows:
![]()
where MW is converted to units from g/mol to µg/µmol, and
1 mL = 103 µL
1 mmol = 103 µmol = 106 nmol
Once the molar concentration is determined, the amount of amino acids in the sample is calculated.
Using the 1-mg/mL protein in Step 1, which required a 27x dilution (5 µL hydrolysate stock + 135 µL buffer) from Section 6.3.1, the nmol in 10 µL of the diluted sample is calculated as follows:

3.3 nmol is well below the 140 nmol limit, so the sample is acceptable.
The HPLC AccQ•Tag Method, commercialized by Waters Corporation in 1992, uses the same pre-column derivatization step as the AccQ•Tag Ultra Method that was introduced in 2006. The AccQ•Fluor reagent, 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC), derivatizes primary and secondary amines in a simple, single-step reaction to yield highly stable, fluorescent adducts. We offer the AccQ•Tag Method as a system package that includes prepackaged reagents and extensive documentation. The AccQ•Tag chemistry package contains the items needed for up to 250 analyses of protein and peptide hydrolysate amino acids.
The AccQ•Tag Derivatization Kit contains five sets of the derivatizing reagents. Each set of reagents includes one vial each of the following:
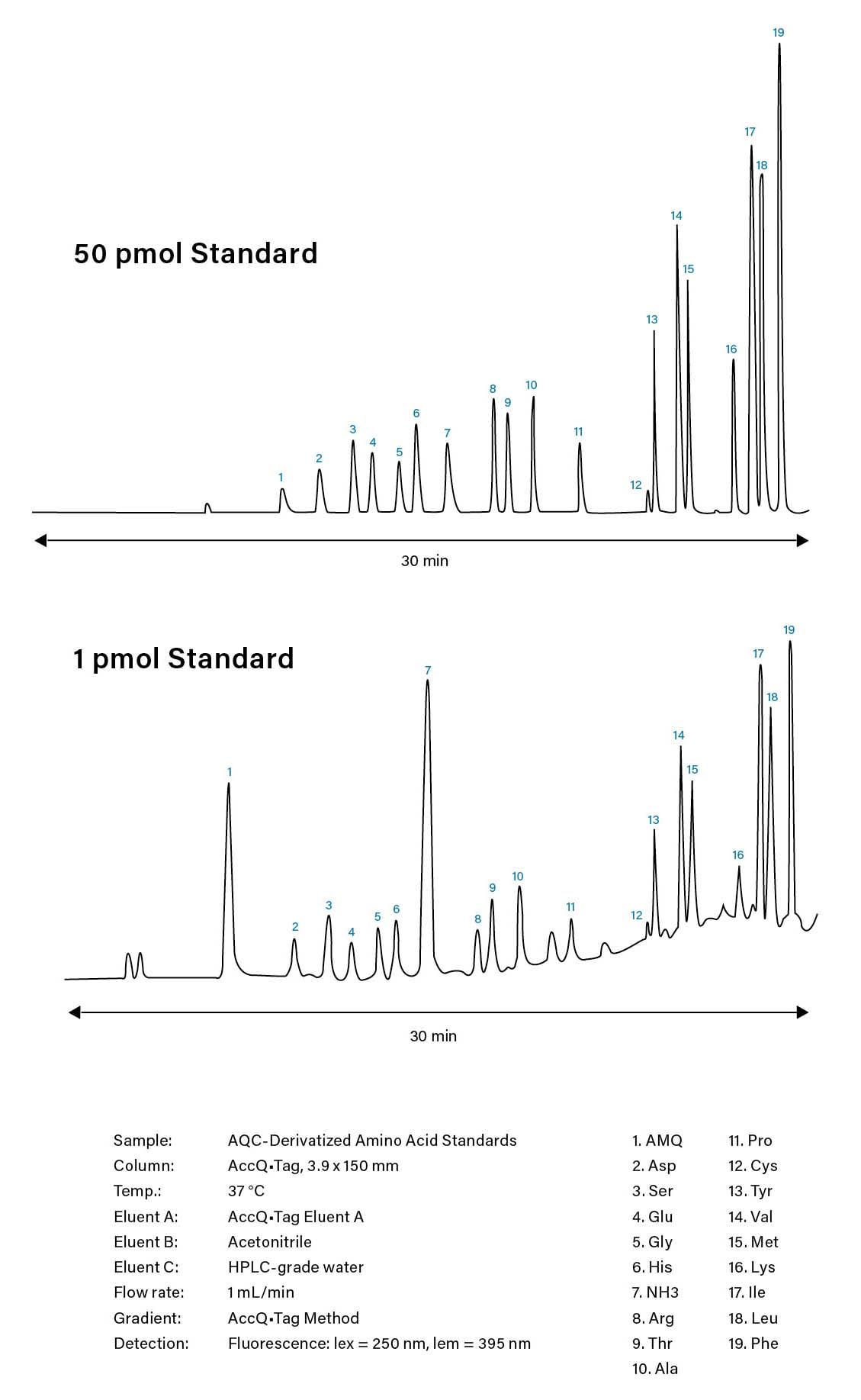
Figure 11. Representative chromatograms of HPLC-based, amino acid analysis using Waters AccQ•Tag Method.
The Waters UPLC Amino Acid Analysis Solution is holistically designed for turnkey amino acid analyses. Pre-column derivatized amino acids are resolved on a Waters ACQUITY™ UPLC System using the included AccQ•Tag Ultra, reversed-phase UPLC column, eluents, and methods. Rugged derivatization chemistry, stable chromatographic baselines, and superior amino acid resolution help ensure accurate, precise, and consistent quantitative results.
The UPLC Amino Acid Analysis Solution includes:
Waters ACQUITY UPLC System supports three different optical detectors: tunable UV, PDA, and fluorescence detector.

Figure 12. Waters UPLC Amino Acid Analysis Solution.
The UPLC Amino Acid Analysis Solution includes two complete methods that use the same instrumentation and chemistries. The first is suitable for the amino acids derived from protein hydrolysates. The second is suitable for the larger number of free amino acids found in process samples such as cell culture or fermentation broths. The methods differ in the dilution of the AccQ•Tag Ultra Eluent A and the separation column temperature. There are no user adjustments of pH or modifications of composition for either Eluent A or Eluent B.
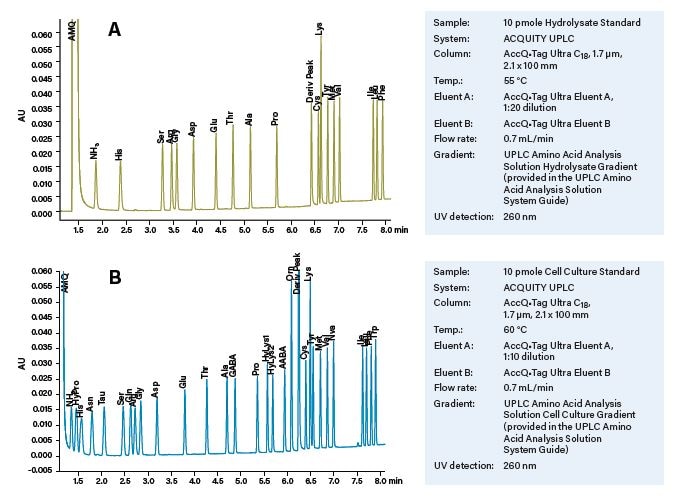
Figure 13. Representative chromatograms of UPLC AccQ•Tag Solutions. (A) Separation of standard amino acids using the UPLC Amino Acid Analysis Solution Hydrolysate Method. (B) Separation of the larger set of standard amino acids using the UPLC Amino Acid Analysis Solution Cell Culture Method. No modifications of the mobile phase or composition is required.
Amino acid analysis of proteins is used both as part of a structural determination and as a measure of the total amount of protein in a sample. The sample is hydrolyzed prior to analysis. For structural analyses, the observed molar ratios of the amino acids are compared to the values expected from the sequence.
For protein amounts, the weights of the amino acids are summed. This measure of protein concentration is used to calculate extinction coefficients where the sample composition interferes with common protein assays. Both weight percent of each amino acid and total protein mass are used to assess the nutritional content of foods and feeds. The Waters UPLC Amino Acid Analysis Solution provides robust, routine analyses across all these applications.
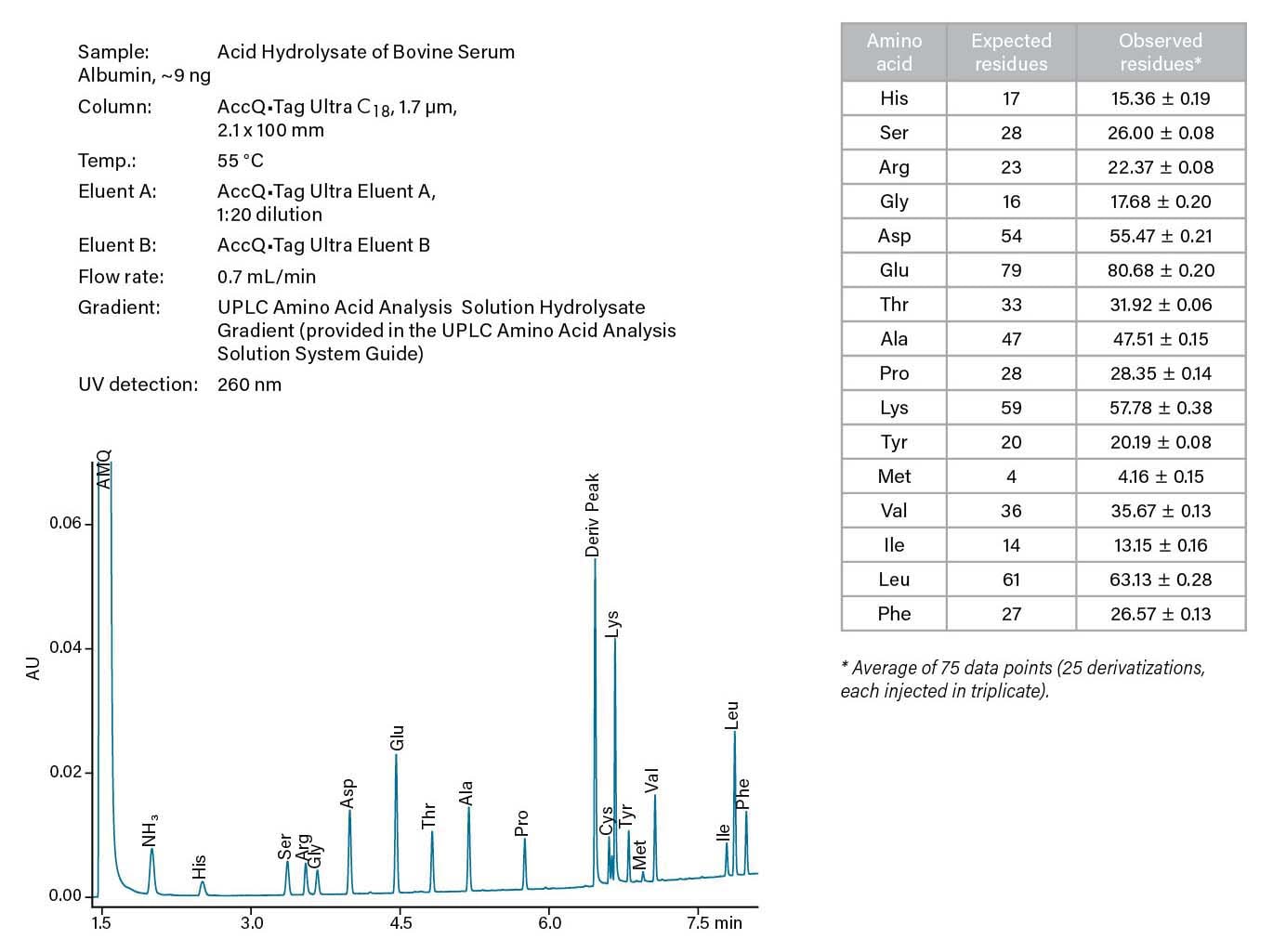 Figure 14. Amino acid analysis of a pure protein hydrolysis.
Figure 14. Amino acid analysis of a pure protein hydrolysis.
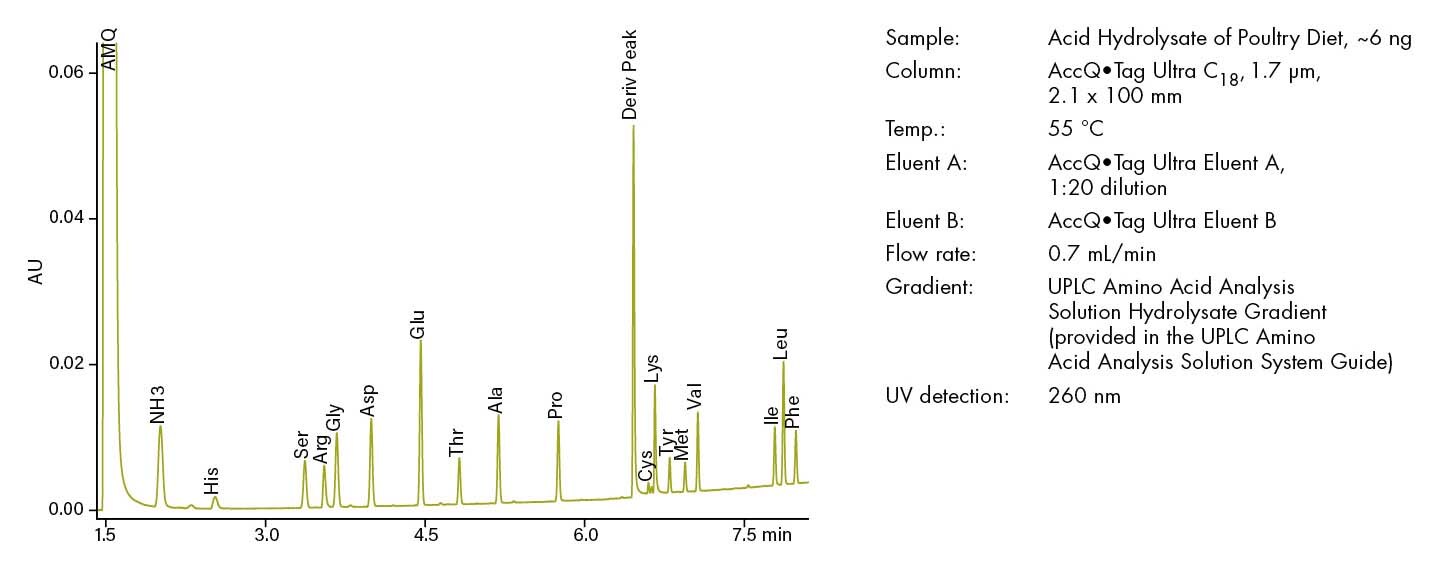
Figure 15. Amino acid analysis of hydrolyzed poultry diet.
| < Previous | Next > |