Hydrolysis of Purified Proteins and Peptides
The phrase “protein and peptide” refers to a relatively pure sample from a bioreactor or purification process. These samples include little or no additional non-protein materials. The generation of free amino acids from an intact protein or peptide is a critical step in the production of useful, accurate amino acid analysis data. To enable this generation, it is necessary to break down or hydrolyze a protein/peptide to its individual amino acid constituents.
In this section, presented are the basic procedures for vapor-phase and liquid-phase protein and peptide hydrolysis, emphasizing the preliminary considerations necessary for optimal analysis.
Additional subsections cover alternative hydrolysis procedures for the analysis of special samples containing amino acids that are not compatible with standard acid (HCl) hydrolysis techniques: tryptophan and cysteine/cystine.
2.1 ACID HYDROLYSIS OF PROTEINS
In this section, we focus on acid hydrolysis by HCl, which is the most common method used in preparing amino acid samples. However, for protein samples to be completely hydrolyzed (by any method), several factors must be considered. The neutralizing buffers, as well as any solids present, should be considered in procedural estimations. Also, the rate or extent of hydrolysis varies across the amino acids present in proteins, necessitating time-course studies of the hydrolysis process and proper validation of the overall methods. Proper sample handling during hydrolysis ensures high-quality results. The most common difficulties in this type of analysis generally stem from improper or sloppy technique. For an effective hydrolysis with this method, four questions must first be answered:
- Is sample dilution necessary?
- What is the sample volume to be dispensed in the hydrolysis tubes?
- What is the specific volume of acid to add to the tubes (liquid hydrolysis only)?
- What is the amount of internal standard, if used during this stage?
The following sections outline the necessary determinations and provide example calculations where appropriate.
2.1.1 Preliminary considerations
Unless sample is limited, there should be between 2 and 25 µg of protein present in the hydrolysis, to minimize the effects of contamination. Regardless of the mode of hydrolysis, 20 µg is recommended.
- There should be no excess of solid material in the sample. This material can interfere with vapor-phase hydrolysis.
- If other solids are present in the sample with the protein, those weights should be added to the protein in the concentration of solids per volume of acid.
- Net concentration of acid in the hydrolysis should be ~6 N.
- Use of an internal standard (e.g., Nva [norvaline]) is recommended over external standards.
- Internal standard should be added prior to hydrolysis. It may be added during the sample preparation, if appropriate, or added to the acid that is then added to the hydrolysis tubes. Typically, the amount of internal standard is calculated to match the standard amount used for calibration.
- Phenol is added to the acid that is used for the hydrolysis, to act as an oxygen scavenger.
2.1.2 Dilution of sample (if necessary) prior to liquid hydrolysis
- Add a minimum of 10 µL of sample (diluted or not) to the hydrolysis tube. Anything less will contribute an unacceptable amount of error due to volumetric uncertainty.
- In the hydrolysis, there also needs to be a weight excess of acid of 10–100 times that of the sample. With an overly concentrated sample, hydrolysis may not work effectively. In these instances, the sample must be diluted. Ensure that there is a ∼100-fold weight excess of acid over solids in liquid hydrolyses.
- Ensure that there is a 25-fold molar excess of acid over buffers in sample. Multiply moles of phosphate buffer by 3 to account for the three titratable groups.
- The total volume for hydrolysis should not exceed 50% of the total capacity of a hydrolysis tube or vessel. For 6 x 50 mm tubes, the recommended maximum volume is 100 µL. Include the acid and internal standard volumes in this final total volume.
➢ Example calculation: Determination of dilution requirement
For a protein that is 5 mg/mL in 150 mM NaCl, the amount of solids (including salts) and the dilution factor will be determined.
Step 1: Determine total solids in sample.
The first step is to determine the total amount of solids in the sample. This can be done by multiplying the protein concentration (µg/µL) by the weight concentration of the salt (concentration x MW) for a total amount of solids.
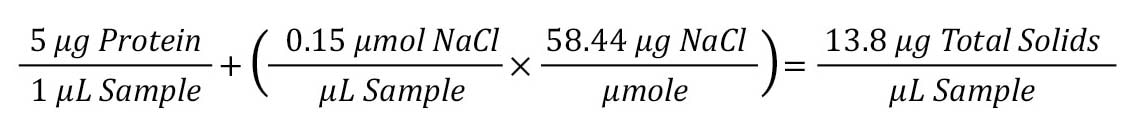
Step 2: Dilution ratio to 20 µg target.
The next step is to determine the dilution needed for the target of 20 µg of protein (in 20 µL). This can be done by multiplying the desired amount/volume by the inverse concentration of the sample. This results in a 1:5 dilution ratio needed for the sample described above.
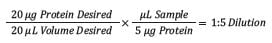
2.1.3 Volume of sample to dispense to the hydrolysis tube
The total recommended volume for a hydrolysis is 100 µL (when using 6 x 50 mm tubes), with the volume of sample ranging from 10–20 µL. This volume of sample, whether diluted or not, depends on the type of hydrolysis performed and follows these guidelines:
- Vapor-phase hydrolysis: vacuum-dry the sample as a thin film on the bottom of the hydrolysis tube.
- Liquid hydrolysis: either dispense the appropriate volume to hydrolyze at least 2 µg of protein in the hydrolysis tube or, from a larger amount of sample (up to 25 µg of protein), reconstitute with enough 0.1 N HCl to transfer ~0.2 µg of protein (ideally in 10 µL) to the hydrolysis.
It is important to note, again, that the lower the amount of protein in the sample aliquot the more vulnerable the analysis may be to contamination.
2.1.4 Volume of acid to add to the hydrolysis
The volume of acid added to the hydrolysis is critical. This is particularly true for liquid- phase hydrolysis. Guidelines for acid volume are below, with an extensive example.
- Vapor-phase hydrolysis:
- Add 200 µL of 6 N HCl with 0.1–0.5% phenol to the bottom of the hydrolysis vessel. (If using a commercial hydrolysis workstation, add the volume recommended by the vendor. For additional information, reference Sections 4 and 5.)
- Liquid-phase hydrolysis (see example below):
- Ensure that the final acid concentration is 6 N (with phenol added).
- Ensure that molar excess of acid is enough (~25-fold) to neutralize buffers.
- Ensure that weight excess of acid over total solids is sufficient (~100-fold). Include the sample matrix and other solids along with the protein in this estimate.
➢ Example calculation: Determine volume of acid to add to a liquid hydrolysis
Following the above guidelines, the minimum amount of acid added for a hydrolysis must exceed 25x the buffer concentration and 100x the weight excess of the sample. Therefore, to determine the volume of acid needed, you must calculate the following:
- Amount of buffer present
- Amount of acid required for neutralization (25x) of the buffer in the sample
- Convert amount of acid to volume
- Total solids in the sample
- Minimum amount of acid required to provide the target excess acid over the sample (100x)
- Convert amount of acid to volume
- Total amount of acid required (sum of neutralization and excess)
For a protein that is 2.1 mg/mL in 2 mM Na/K2PO4:
Step 1: Determine the amount of buffer in each tube.
The amount of buffer in each tube is determined by multiplying the buffer molar concentration by the number of titratable groups (three for the phosphate buffer) and then adjusting to the total volume of the sample dispensed—in this case, 10 µL.
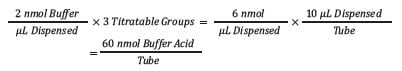
Each tube will contain 60 nmol of buffer.
Step 2: Determine the amount of acid required for a 25-fold excess of the buffer.
Next, the 25-fold excess is determined by multiplying the amount by 25.
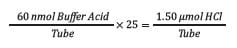
This number is the moles of buffer acid required for effective neutralization.
Step 3: Determine the volume of 6 N HCl needed for each tube for neutralization of sample buffer (convert moles to volume).
The moles of buffer required for neutralization then must be converted to a volume of acid to add to each tube.
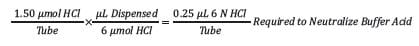
For this sample, a volume of 0.25 µL will effectively neutralize the buffer.
Step 4: Determine the total solids (protein + buffer) in each tube.
To achieve the 100x excess of acid over the sample, the total amount of solids in the sample must be determined. Total solids will be the protein plus buffer plus any other material present. In this case, the sample is a purified protein.
This determination can be made by multiplying the protein concentration (µg/µL) by the weight concentration of the salt (concentration x MW) for a total amount of solids.

The total solids for this sample calculate to be 24.9 µg.
Step 5: Calculate the total HCl in a tube for 100-fold excess of acid.
To determine the target 100-fold excess of acid, multiply the total solids by 100.
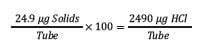
Step 6: Convert the excess of acid to volume of 6 N HCl for acid excess in each tube.
Finally, convert the weight excess to a volume of 6 M HCl to add to each tube by multiplying the weight by the weight per mole for the acid by the molarity of the acid.
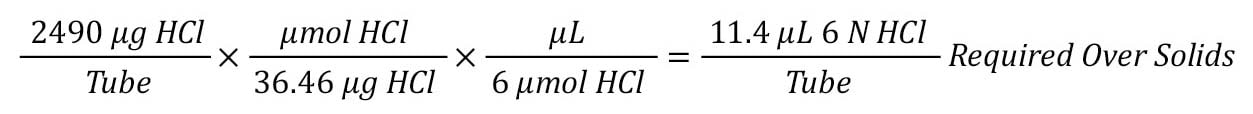
In this case, 11.4 µL of 6 M HCl will provide a 100-fold excess of acid over the sample.
Step 7: Final volume of 6 N HCl to add to each tube (Step 3 + Step 6).
The final volume of acid to add to each tube is the sum of the volumes needed to neutralize the sample buffer and provide the 100-fold excess:
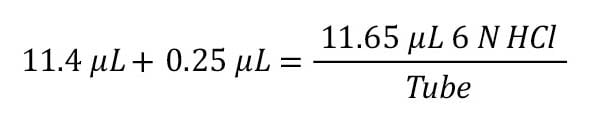
A volume of 11.65 µL of 6 M HCl is needed to ensure an effective hydrolysis. This volume can be rounded up to a volume that can be transferred accurately.
2.1.5 Internal standard (IS)
The use of an internal standard (IS) best compensates for variable hydrolysis of the individual amino acids of the sample. Norvaline (Nva) is a commonly used internal standard.
When using an internal standard:
- It can be prepared in and dispensed with the sample.
- It can be added separately from the sample.
- It can be prepared in and dispensed with the acid.
- Make certain to prepare the internal standard so that the 1 µL injection volume on the instrument delivers the same amount on column as the sample.
To determine the amount of IS needed in the starting sample, the calculation works back from the desired amount of IS needed in the sample. As part of this calculation, it’s important to consider the derivatization step.
➢ Example calculation: Determine amount of internal standard for a sample
Step 1: Determine total IS needed per tube.
The total IS is determined by, working backward, multiplying the amount of IS required in the final sample, the dilution factor of the derivatization, and the reconstituted sample in the hydrolysis tube. In this example, 25 pmol of IS is needed in the final derivatized sample, with the sample being diluted 10x during the derivatization and 5x dilution of the sample prior to derivatization. Thus:
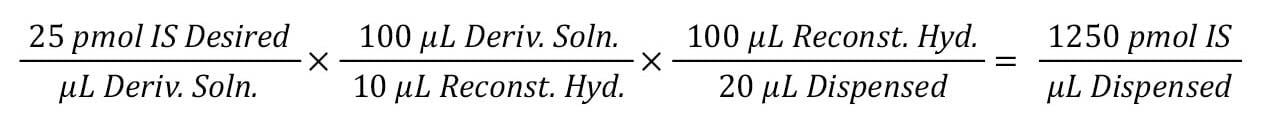
This indicates we need 1250 pmol of IS in our hydrolysis sample.
Step 2: Convert molar concentration of IS to weight.
Given the MW (mg/mol) and conversion from µL to mL, we can see that we need 0.14 mg of IS added to each mL of our starting sample.
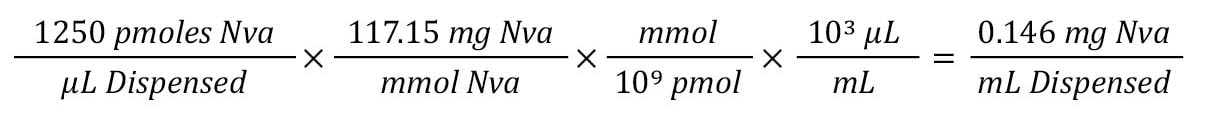
2.1.6 Vapor-phase acid hydrolysis
Vapor-phase hydrolysis is recommended for relatively pure protein or peptide samples containing little or no particulate material. It’s considered the most sensitive approach. A stand-alone, automated, hydrolysis workstation is preferred for this type of hydrolysis. The vacuum control, temperature maintenance, nitrogen flushes, and sample drying required in the procedure are best performed by an automated system such as the Eldex hydrolysis workstation described in Section 4.
Reagents:
- 6 N HCl with 1% phenol by volume
- Nitrogen (pre-purified grade)
- Dry ice
Note: For additional reagent information, consult the hydrolysis workstation’s operational manual.
Procedure (based on using an automated workstation):
- Dry an aliquot containing 0.5–20 µg of protein in a 6 x 50 mm hydrolysis tube.
- Add 200 µL of constant-boiling HCl containing 0.5% phenol to the bottom of the vacuum vial (see tip below).
- Seal the vial under vacuum after three alternate vacuum-nitrogen flushing steps.
- Hydrolyze at 112–116 °C for 24 hours.
- Cool the vial; remove excess HCl from the outside of tubes by wiping with a laboratory tissue; dry under vacuum.
|
TIPS:
- Use crystalline phenol and place one crystal (≈0.5 mg) in the bottom of the vial. The crystal is cleaner and more stable than liquified phenol.
- Carefully pipet the sample and the internal standard into the bottom of the tubes; use a syringe for accuracy and ease of delivery.
- You can label the tubes by scoring them with a file or diamond-tip pencil.
- Prevent droplets of HCl from draining into the tubes. Keep tubes upright in the vacuum vial. With fewer than 10–12 tubes, add blank tubes for support. Slightly tilting the vial during cooling can be helpful.
|
Note: A brown color in the HCl is common after hydrolysis. It derives from the phenol. Ethanol or acetone works well to remove discoloration from vials and tops.
CAUTION: Hydrolysis problems may be difficult to distinguish from derivatization problems.
2.1.7 Liquid-phase acid hydrolysis
Liquid-phase hydrolysis is used when the samples are more complex. In this case, particulate or other foreign matter is present that may interfere with the vapor-phase process. This approach is considered less sensitive overall; but, when performed carefully and precisely, can give good results.
- Apparatus and reagents needed for this methodology:
- Analytical balance
- Oven or heating block capable of maintaining set temperature ±0.1 °C
- Vortex mixer
- Volumetric pipettes
- Adjustable micropipettes
- Hydrolysis tubes with caps, 6 x 50 mm recommended
- Nitrogen source for flushing
- 6 N HCl
Procedure:
Before starting –
Either weigh samples corresponding to approximately 20 mg protein to the nearest 0.1 mg into hydrolysis tubes or transfer diluted sample to tubes (as calculated in Sections 2.1.3 and 2.1.4). Normally, a total volume of 10 µL is usual. Mix.
- Precisely add the correct volume of internal standard, calculated from Section 2.1.5, to the hydrolysis tubes. Mix.
- Add an excess of 6 N HCI (calculated from Section 2.1.4, Step 3), mix, and purge with nitrogen for 30 seconds. Cap immediately.
- Place in oven at 110 °C for 24 hours. (These parameters should be the result of a time-course study for the protein and amino acids of interest.)
- Remove from oven and allow to cool.
- Sample is ready to move to the derivatization step.
2.1.8 Acid hydrolysis troubleshooting
Several amino acids are affected by improper hydrolysis. For example:
- Low yields of methionine (Met) and tyrosine (Tyr) are usually symptomatic of a poor hydrolysis procedure—either impure HCl or inadequate removal of oxygen. Either issue can lead to formation of chlorine, and subsequent chlorination of Tyr.
- Low yields of the hydrophobic amino acids (Ile, Leu, Val, and others) may indicate incomplete hydrolysis, caused by low oven temperature, shortened hydrolysis time (particularly if a fast, high temperature hydrolysis is being used), or loss of HCl resulting from over-evacuation after purging with nitrogen (this may be a sample-specific problem; some sequences, such as lle-Val-Leu, are extremely resistant to hydrolysis). Verify that liquid HCl remains visible in the vial before placing it in the oven. Too much HCl can also be a problem; liquid can condense in sample and result in a brown residue, loss of Tyr and Met, and appearance of stray peaks.
- If a gas-phase hydrolysis workstation is not being used, ensure that the setup for hydrolysis is adeaquate. The entire hydrolysis vial must be encloded in the over; use of a heating block that encloses only the bottom half of the vial will result in condensation of liquid HCl at the top, and hydrolysis will be ineffective.
2.2 ANALYSIS OF TRYPTOPHAN (Trp)
Analysis of Trp in proteins and peptides is complicated by the instability of this amino acid under normal hydrolysis conditions with 6 N HCl. Alternative hydrolysis procedures can be used to generate intact Trp for analysis:
- Sulfonic acid hydrolysis (e.g., methane sulfonic and p-toluene sulfonic acids)
- Base hydrolysis and the use of thiol reagents in HCl, such as B-mercaptoethanol (BME) or thioglycolic acid
2.2.1 Methanesulfonic acid (MSA) hydrolysis for tryptophan analysis
The MSA reagent must be added directly to the 6 x 50 mm sample tubes (liquid- phase hydrolysis), because the acid is not volatile. The addition of methanol and neutralization after hydrolysis provides good results for tryptophan and does not interfere in any subsequent derivatization process. Figure 2 illustrates the reaction of MSA with protein.
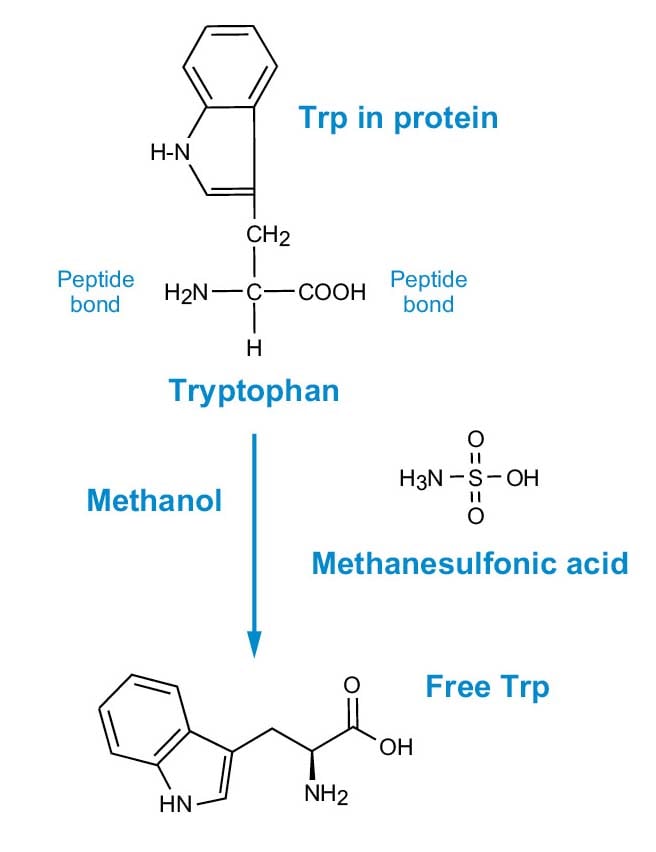
Figure 2. MSA hydrolysis for Trp analysis.
Note: This procedure can also be used for determination of Cys and Met. It converts Cys to Cya and Met to methionine sulfone.
Note: Tyr and Trp are not stable in the performic acid oxidation procedure traditionally used for Cys and Met analysis.
2.2.1.1 Apparatus and reagents
- 4 M MSA containing 0.2% (w/v) tryptamine HCl
- Ultrapure water
- Methanol-water-triethylamine, 2:2:1
- Hydrolysis tubes, 6 x 50 mm
- Vacuum drying apparatus
- Water bath or heating block
2.2.1.2 Procedure
- Add 20 µL of 4 M MSA containing 0.2% (w/v) tryptamine HCl to each 6 x 50 mm sample tubes containing dried sample.
- Add I00 µL of water to the reaction vial.
- Seal for hydrolysis.
- Hydrolyze at 110 °C for 20–24 hours.
- Cool, open vial, and add 22 µL of 4 M KOH (enough to neutralize) to each sample tube.
- Dry under vacuum.
- Sample is ready for derivatization.
|
TIPS:
Check for interferences with control blank samples.
- Use fresh, pure MSA.
- It may be that some of the interference occasionally obscuring Tyr or Val comes from the tryptamine that is used as an additive in the commercial acid. Use of MSA without the additive could be a solution.
- Use fresh KOH (or substitute NaOH, if you find it is cleaner in your lab). Test the ability of the base to neutralize the acid on a trial sample. Add a volume of base that keeps the excess of base to a minimum.
- Make a Trp standard 2.5 µmol/mL in H20. Store in the freezer. Mix Trp standard 1:1 with H standard for calibration use.
WATCHOUTS:
■ Interference with Tyr and Val can occur. The cause is unknown. (HCl is preferable for the hydrolysis of Tyr.)
■ Low Met yields are a function of the hydrolysis conditions.
■ Yields of Trp and Met are nonlinear due to the hydrolysis process, not the analysis procedure. As the amount hydrolyzed is reduced, the yields fall; with Met the result is more dramatic.
■ Poor reproducibility of Arg results—cause unknown.
|
2.2.2 Alkaline hydrolysis for tryptophan analysis
If acid hydrolysis of tryptophan raises stability issues, hydrolysis of protein using base is another alternative.
2.2.2.1 Apparatus and reagents
- NaOH
- Acetic acid
- Ultrapure water
- Hydrolysis tubes, 6 x 50 mm
- Vacuum drying apparatus
- Water bath or heating block
2.2.2.2 Procedure for base hydrolysis
- Use of plastic (e.g. Teflon®) tubes may be required to prevent formation of silicates and subsequent solubilization or derivatization problems.
- Add 20 µL fresh 4 M NaOH directly to the hydrolysis tube as in the MSA procedure above.
- Seal the tube and heat at 112 °C for 16 hours.
- Cool and neutralize with excess acetic acid.
- Sample is ready for derivatization.
- Run blanks to determine the contamination level.
- Verify the method on standards and known samples.
2.3. HYDROLYSIS METHODS FOR ANALYSIS OF SULFUR-CONTAINING AMINO ACIDS (CYSTEINE, CYSTINE, AND METHIONINE)
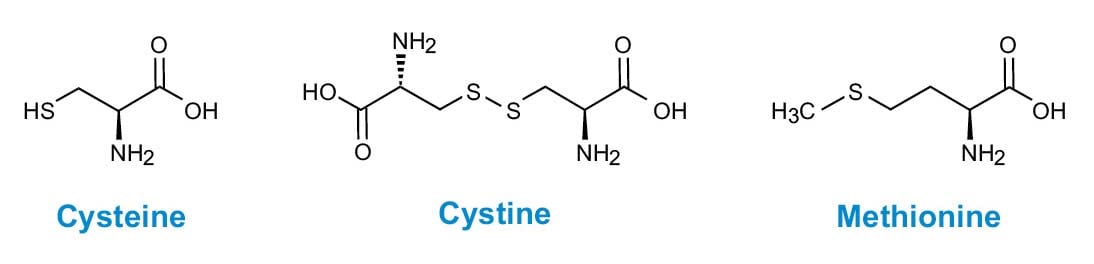
Figure 3. Sulphur-containing amino acids.
The quantitation of cysteine (Cys) in protein samples is complicated by the instability of this amino acid in standard acid hydrolysis conditions. Unfortunately, unlike with Trp, alternative acid or base hydrolyses are not satisfactory. Two common procedures for Cys analysis involve conversion of the cysteine to more stable derivatives. The first procedure is alkylation of the sulfhydryl group, and the second is an oxidation to the acid-stable sulfonic acid, cysteic or cyanuric acid (Cya).
WARNING: A further complication in Cys analysis is that much of the amino acid is present as the dimer, cystine (Cys2), which must be reduced to cysteine prior to any alkylation procedures.
It is important to note that these specific procedures are performed prior to the standard acid hydrolysis step.
2.3.1 Performic acid oxidation for deamidation of cysteine, cystine, and methionine
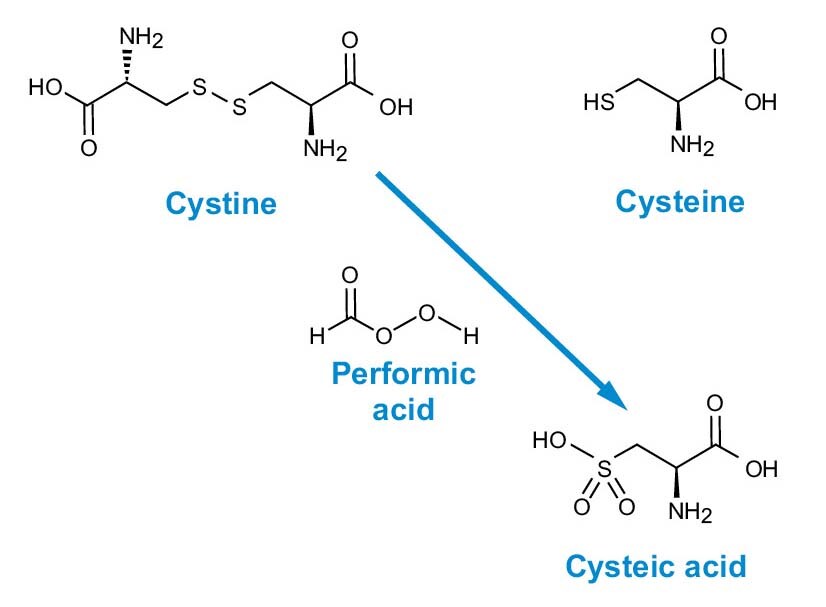
Figure 4. Performic acid oxidation of cystine and cysteine to cysteic acid.
Performic acid is a powerful oxidizing reagent that quantitatively converts cysteine (Cys) and cystine (Cys2) to cysteic acid (Cya) (Figure 4). Literature contains many references to the use of this reagent in a wide variety of conditions and procedures. The following procedure is based upon that of Tarr, G.E., 1986.
Note: This procedure yields the most accurate results for cysteine and methionine.
2.3.1.1 Apparatus and reagents
- Vacuum drying apparatus
- 6 x 50 mm hydrolysis tubes with caps
- Ultrapure formic acid
- Ultrapure hydrogen peroxide
- Analytical balance
- Micropipettes
2.3.1.2 Procedure
- Vacuum-dry the sample (0.1–10 µg protein or 50–2000 pmol peptide) in a 6 x 50 mm hydrolysis tube.
- Mix 19 volumes of 97% formic acid with 1 volume of hydrogen peroxide; let stand covered one hour at 22 °C.
- Add 10 µL of this reagent to the dried sample, let stand 30 minutes at 22 °C, and vacuum-dry.
- Hydrolyze using the standard 6 M HCl procedure (See Section 1.1).
Note: Tyr and Trp are not stable in this oxidation procedure.
2.3.2 Alkylation of cystine
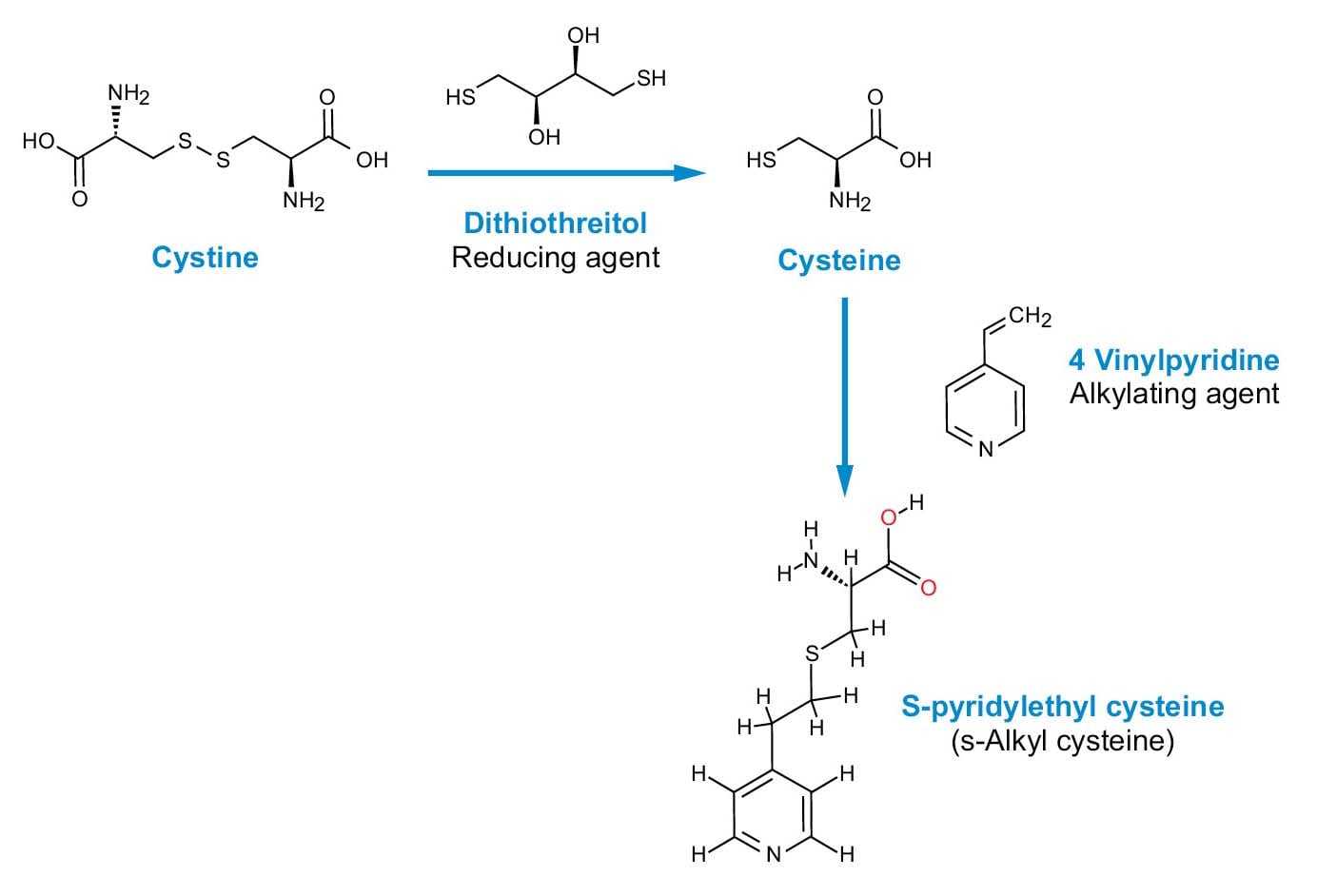 Fiugre 5. Alkylation of cystine and cysteine.
Fiugre 5. Alkylation of cystine and cysteine.
Procedures that alkylate are more selective than performic acid oxidation and result in little or no change to the other amino acids. This makes them better suited to the analysis of the complete protein, as well as other procedures that may follow Cys modification, such as peptide mapping.
In this method, alkylation is presented with 4-vinylpyridine; the general procedure outlined below can also be adapted for several alkylating agents. In principle, a sufficient, reducing reagent must be added to the sample to convert cystine (Cys2) to cysteine (Cys). This is followed by an addition of excess alkylating reagent to the reduced sample (Figure 5).
2.3.2.1 Apparatus and reagents
- Vacuum dryer, nitrogen drying source, or lyophilizer
- Resealable vials
- Analytical balance
- pH meter
- Micropipettes
- Pyridylethyl cysteine (PEC) as standard
- Guanidinium HCl (Gu-HCl)
- Dithiothreitol (DTT)
- 4-vinylpyridine (4-VP)
- N-ethylmorpholinium acetate
- Ultrapure acetic acid
- Amino Acid Standard (p/n: WAT088122)
2.3.2.2 Reagent preparation: 0.5 M, pH 8.3
- Add water to 6.4 mL of N-ethylmorpholinium to bring the volume to 100 mL.
- Titrate to pH 8.3 with acetic acid.
2.3.2.3 Procedure
The following procedure can be used for 1–1000 nmol of protein or peptide.
- Place sample in a sealable vial; vacuum dry, N2, or lyophilization.
- Dissolve sample in 1 mL buffer.
- Add 1 g Gu-HCl. Mix.
- Add 4 mg DDT. Mix.
- Blanket sample with N2, seal tightly, and incubate at room temperature for 4 hours.
- Add 8 µL 4-VP, blanket with N2, seal, and incubate at room temperature for 4–16 hours.
- Add 3 mL water.
- Desalt.
- Sample can now be acid hydrolyzed.
Note: The total reaction volume can be scaled down by reducing buffer to 0.25 mL, Gu-HCl to 250 mg, DTT to 1 mg, and 4-VP to 2 µL. This is adequate for up to 250 pmol of sample. After alkylation, dilute with 750 µL H20 and proceed.
2.3.2.4 Calibration standard for subsequent derivatization (optional)
- Make a 2.5 mM solution of pyridylethyl cysteine (PEC).
- Add 200 µL of Amino Acid Standard to 200 µL.
- Derivatize 10 µL of combined PEC calibration mixture.
- Reconstitute in 100 µL; inject 4 µL to calibrate at the 250 pmol level.
Note: 8 µL of 4-VP is approximately 74 µmol. Substitution of other alkylating reagents for 4-VP (e.g., iodoacetic acid) in the procedure can be made using the same reagent concentration.
![]()
![]()
![]()
![]()
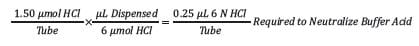
![]()
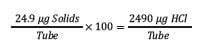
![]()
![]()
![]()
![]()



 Fiugre 5. Alkylation of cystine and cysteine.
Fiugre 5. Alkylation of cystine and cysteine.