Although this guide generally concentrates on the initial steps of amino acid analysis— emphasizing sample preparation for hydrolysis—this section presents a brief discussion of the more common quantitation approaches used in amino acid analysis. In these examples, the results from amino acid analysis have been obtained; the original sample concentration needs to be calculated. These examples assume that AccQ•Tag or AccQ•Fluor Reagents have been used for the derivatization.
Before proceeding with quantitation:
For some analyses, the goal is simply to determine the concentration of the analyzed sample. These results are usually expressed in molar concentrations, such as µmol/L. The chromatographic results are typically given in picomoles. To determine the concentration, the picomoles of amino acid reported by the chromatographic software is divided by the injection volume. This value is then multiplied by the volume of diluent divided by the volume derivatized. To complete the calculation, multiply by the dilution factor, and then convert the units to µmoles per liter.
To determine the concentration of the amino acid in the original sample, based on the reported value, we use the following equation:
![]()
Where:
pmol AA = the amount reported for amino acids in sample Vi = injection volume in µL, typically 1 µL
Vd = volume derivatized in µL, typically 10 µL
Vr = volume of diluent used to reconstitute sample in µL Dil. Factor = dilution factor
Example:
An initial 100-µL sample of hydrolyzed protein sample was diluted 1:1 with internal standard. A 10-µL aliquot was derivatized. The injection volume was 1 µI and the reported value was 312.5 pmoles for asparagine (Asn).
The Asn concentration in µmol/L would be:
![]()
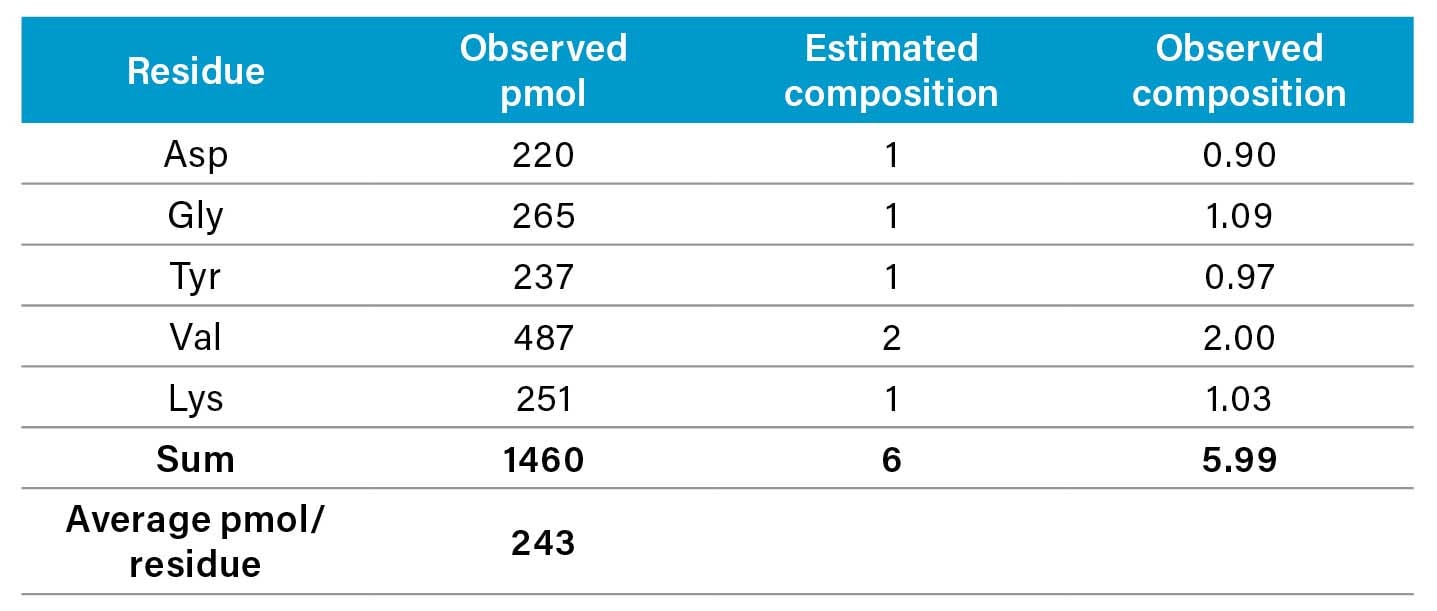
Determination of the amino acid composition of a hydrolyzed protein involves calculating the molar ratios of the components within a sample.
Each pure protein has a specific stoichiometric number of residues for each amino acid it contains. Ideally, the results of an analysis yield molar ratios that are integers. The degree to which the observed values approach this ideal is a function of the purity of the protein or peptide, the adequacy of the hydrolysis conditions, and the quality of the amino acid analysis.
WARNING: For larger proteins, estimation of the number of residues is more difficult because of the greater precision required.
WARNING: Recovery of some amino acids from hydrolysis may be variable (Ser, Tor, Tyr, and Met are subject to degradation; Val and Ile bonds may be difficult to cleave).
NOTE: To improve quantitation in such cases, it is recommended that a time-course study is performed. Typically, samples are hydrolyzed for 24, 48, and 72 (or 96) hours; labile amino acid values are determined by extrapolation back to zero time, while the values for Ile and Val are taken from the longest hydrolysis.
In some cases, as noted earlier, calculation of the mole percent (the number of residues of each amino acid per 100 residues of protein) may be satisfactory. This is determined as follows:
![]()
If an estimate of the molecular weight of the protein is available, two approaches can be used to calculate an approximate composition:
This alternative procedure requires more knowledge about the sample.
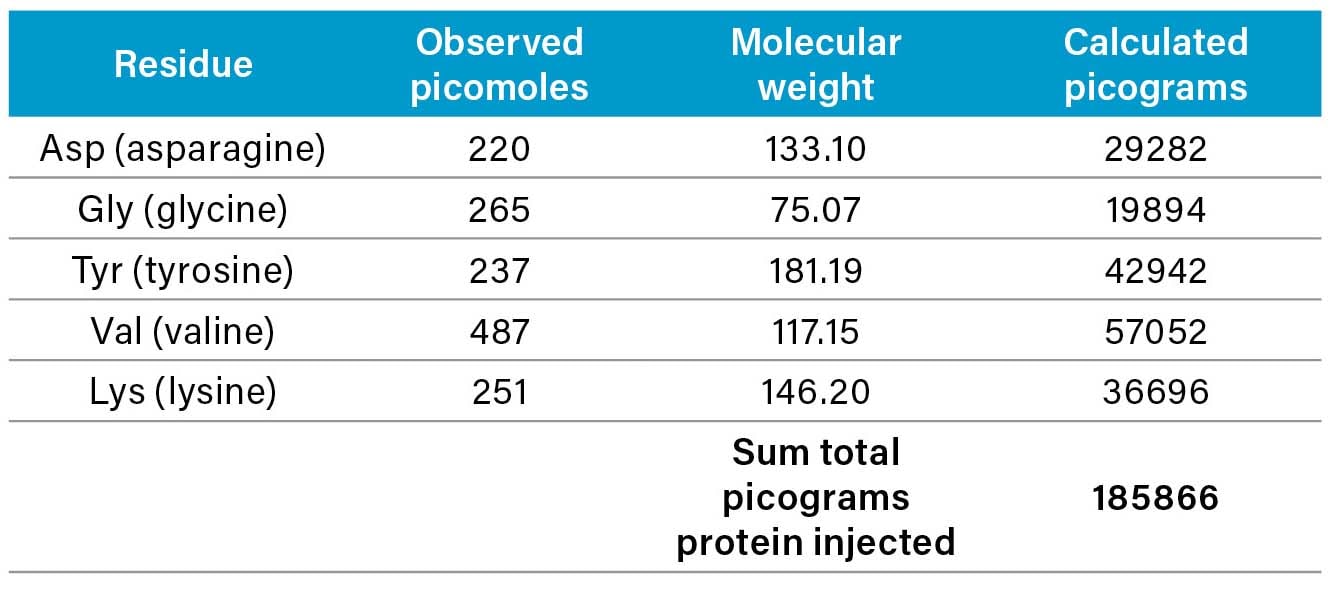
The concentration of peptide or protein in the original sample can be calculated from the sum of the products of the number of picomoles of each amino acid multiplied by its corresponding molecular weight.
Using the example cited in the table from Section 7.3.1, the calculation begins as follows:
![]()
For Asp (asparagine) in the sample: 220 picomoles observed with a molecular weight for the amino acid of 133.10 g/mole gives the following:
![]()
This same calculation is applied for each desired amino acid. The table below shows the calculated picograms for each amino acid, with the sum total of picograms protein injected from the sample in the lower right.
In the analysis of food and feeds, the above-mentioned calculated values also apply. However, the more important information for most feed analyses is the content of specific, growth-limiting amino acids, such as methionine and cysteine. The value most commonly of concern is the % amino acid content by weight in the sample.
To calculate the % by weight of an amino acid:
Step 1: Convert reported molar amino acid concentration to reported weight (g/mL).
The reported value of Amount (pmol/µL) must be multiplied by the Residue MW (gm/mol) and any conversion factors.
![]()
The reported amount in g/mol is then multiplied by any dilution factors. The result is then divided by the weight of the sample, and then multiplied by 100 to convert to a percent.
![]()
Where:
Amount = amount of amino acid in g/mL
Dilution = dilution of sample
Sample weight = weight in mg
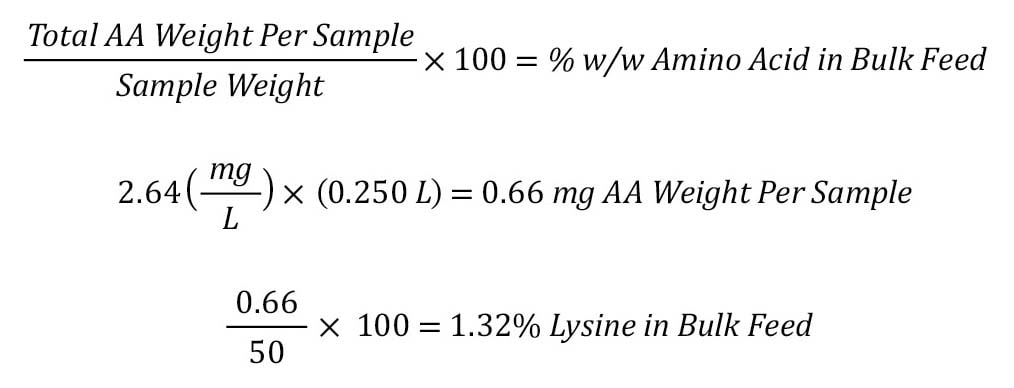
This method requires the use of an internal standard.
Step 1: Calculate a response factor (RFaa) for each amino acid.
The peak area for the internal standard is multiplied by the weight of the amino acid in mg. This is then divided by the result of multiplying the peak area of the amino acid by the weight of internal standard.
![]()
Where:
RFaa = response factor for the amino acid
Pn = peak area for internal standard
Paa = peak area for the amino acid in the sample
Waa = weight of the amino acid in mg
Wn = weight of internal standard in mg
![]()
The % content calculation begins with the multiplication of the peak area of the amino acid by the calculated response factor. This is then multiplied by the internal standard factor. This number then is divided by the multiplicand of the peak area of the internal standard and the weight of the sample analyzed. This is then converted to a percent.
![]()
Where:
Paa = peak area of amino acid
Pn = peak area of internal standard
RFaa = calculated response factor
IS = calculated internal standard factor
For an amino acid in a feed sample, the following values were determined:
Weight of amino acid (Waa) = 0.5 mg
Peak area of amino acid (Paa) = 100,000
Peak area of internal standard (Pn) = 110,000
Weight of internal standard (Wn) = 0.5 mg
Weight of test portion (Ws) = 10 mg
Step 1: Calculate the response factor, RFaa:
![]()
Step 2: Calculate the internal standard factor, IS:
0.5 mg x 2 x 10-2 = 0.05
Step 3: With both values calculated, the % amino acid can be determined:
![]()
The amino acid of concern is present at a level of 0.9% by weight of the feed.
| < Previous | Next > |