A column tube and fittings must contain the chromatographic packing material [stationary phase] that is used to effect a separation. It must withstand backpressure created both during manufacture and in use. Also, it must provide a well-controlled [leak-free, minimum-volume, and zero-dead-volume] flow path for the sample at its inlet, and analyte bands at its outlet, and be chemically inert relative to the separation system [sample, mobile, and stationary phases]. Most columns are constructed of stainless steel for highest pressure resistance. PEEK™ [an engineered plastic] and glass, while less pressure tolerant, may be used when inert surfaces are required for special chemical or biological applications. [Figure M-1].

Figure M-1: Column Hardware Examples
A glass column wall offers a visual advantage. In the photo in Figure M-2, flow has been stopped while the sample bands are still in the column. You can see that the three dyes in the injected sample mixture have already separated in the bed; the yellow analyte, traveling fastest, is just about to exit the column.
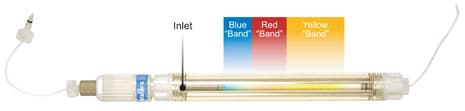
Figure M-2: A Look Inside a Column
Separation Performance – Resolution
The degree to which two compounds are separated is called chromatographic resolution [RS]. Two principal factors that determine the overall separation power or resolution that can be achieved by an HPLC column are: mechanical separation power, created by the column length, particle size, and packed-bed uniformity, and chemical separation power, created by the physicochemical competition for compounds between the packing material and the mobile phase. Efficiency is a measure of mechanical separation power, while selectivity is a measure of chemical separation power.
Mechanical Separation Power – Efficiency
If a column bed is stable and uniformly packed, its mechanical separation power is determined by the column length and the particle size. Mechanical separation power, also called efficiency, is often measured and compared by a plate number [symbol = N]. Smaller-particle chromatographic beds have higher efficiency and higher backpressure. For a given particle size, more mechanical separation power is gained by increasing column length. However, the trade-offs are longer chromatographic run times, greater solvent consumption, and higher backpressure. Shorter column lengths minimize all these variables but also reduce mechanical separation power, as shown in Figure N.
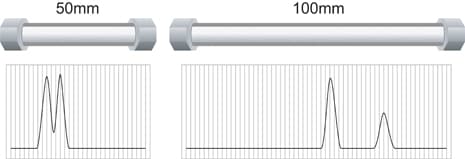
Figure N: Column Length and Mechanical Separating Power [Same Particle Size]
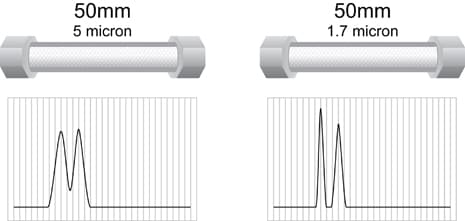
Figure O: Particle Size and Mechanical Separating Power [Same Column Length]
For a given particle chemistry, mobile phase, and flow rate, as shown in Figure O, a column of the same length and i.d., but with a smaller particle size, will deliver more mechanical separation power in the same time. However, its backpressure will be much higher.
Chemical Separation Power – Selectivity
The choice of a combination of particle chemistry [stationary phase] and mobile-phase composition—the separation system—will determine the degree of chemical separation power [how we change the speed of each analyte]. Optimizing selectivity is the most powerful means of creating a separation; this may obviate the need for the brute force of the highest possible mechanical efficiency. To create a separation of any two specified compounds, a scientist may choose among a multiplicity of phase combinations [stationary phase and mobile phase] and retention mechanisms [modes of chromatography]. These are discussed in the next section.
| < Previous |
Next >
|