How a LC Instrument Was Modified To Accommodate CO
As discussed in the previous chapter, CC closely resembles LC from a separations standpoint. Even from an instrument point of view, CC resembles a LC system (see Figure 9) in all respects except for an additional component, the ABPR, which pressurizes the entire system above a set point. To harness the advantages of CO2-based mobile phase and give it the modern benefits of UPLC, Waters modified its low-dispersion, pressure-tolerant ACQUITY UPLC system by making key system components compatible with a compressible solvent like CO2. Most notably this included the liquefied CO2 pump for metering the flow through the CC system. Although the CO2 is first liquefied, (e.g. at 13o C) it is almost three times more compressible than methanol or acetonitrile. Therefore the pump had to be modified for reproducible convergence chromatography.
Challenges of past analytical SFC systems
Historically, analytical SFC systems were notoriously unreliable. All of them were repurposed LC systems. Pumps, injectors and detectors designed for LC weren’t meant to work with compressed CO2.
Single-stage reciprocating LC pumps couldn’t compress and deliver CO2 accurately, repeatedly and reliably. They are not designed to work with a liquid as compressible as CO2, causing variable mobile phase mass-flow rates as well as mass-compositions. This changes the solvation power of the mobile phase and often shows up as shifting retention times from injection to injection or system to system.
Highly compressible CO2-based mobile phases also compromise analytical sensitivity due to noise generated by the pump and the back-pressure regulator. Also, due to significant problems of poor accuracy and precision using partial loop injections, repurposed HPLC instruments are often restricted to full loop injections, limiting injection volume choice. At the systems level, repurposed HPLC systems feature a significantly greater system dispersion volume which causes unwanted band-spreading, preventing the use of more efficient 1.7 mm particle columns. These drawbacks greatly limit the potential throughput and achievable performance of a repurposed LC CO2 -based system.
Next, we take you through a review of the innovations made to each individual module of the ACQUITY UPC2 System (Figure 9).
Solvent Management Technology (The Pump)
To ensure the accurate and precise control of mobile phase flow rates and composition we need to look at the whole flow path of the system. As we’ve pointed out before, repurposed HPLC pumps, which are designed to both compress and accurately deliver the specified volume of solvent at the same time, are incapable of handling a fluid as compressible as liquid CO2. On some SFC instruments incoming CO2 passes through a device for pre-compression and chilling. This device sits alongside the chromatographic system (Figure 10). The farther away from the pump this device sits, the more difficult it becomes to accurately control the CO2 mass-flow rate, as CO2 density can change between the pre-compression and the pumping step, depending on the ambient temperature. In addition, the pumping algorithms (internal control software) of traditional SFC systems, generally designed to deliver relatively incompressible liquids, struggle to maintain compositional accuracy, precision, and retention time reproducibility. The same problems occur when trying to reliably deliver low percentages of co-solvent (less than 5%), making it difficult to analyze mixtures with diverse polarity.
In contrast, the Waters ACQUITY UPC2 Binary Solvent Manager (BSM) is specifically designed to manage compressible fluids with a fully integrated pre-compression device for exceptional control of the mass-flow rate and mass-composition resulting in reliable, reproducible retention times and negligible baseline noise. As stated previously, in a compressible fluid system, solvent density controls mobile phase solvating power, so precise control is critical for reproducibility. Separate control algorithms, essential for compressible and non-compressible liquid components, accurately blend different mobile phase compositions, including low percentages of co-solvent (Figure 11), and delivering reproducible gradient profiles (Figure 12).
Analytical SFC systems have never before achieved such fine levels of control, especially for gradient separations. The ACQUITY UPC2 System is designed to precisely control pump intake, compression, and delivery, giving it the reproducibility expected from an Ultra Performance LC. The volumetric density control utilized within the ACQUITY UPC2 BSM surpasses mass flow control giving exceptional chromatographic precision. This leads to controlled elution times and exceptional solvation strength control. The pump heads themselves are independently cooled, improving density control of CO2 and hence accurate mass delivery. The pump and integrated compression algorithms are so effective and the control so precise, that either liquid or gaseous CO2 can be used as the initial mobile phase. Figure 13 shows the inner workings of the BSM. The co-solvent pump is a UPLC pump, whereas the CO2 pump is behind the insulated black cover. Since the compression and chilling device is integral to the pump, this insulated cover enables more precise density control of incoming CO2.
Sample Injection
Traditional analytical SFC systems – whether they use full loop or partial loop injectors - struggle to reproducibly inject low volumes of sample. In most cases, only full loop injections are possible; with partial loop injections it is difficult to maintain the injection solvent homogeneity. Accuracy, precision, and linearity therefore suffer, preventing analyte quantitation. Large amounts of sample may be wasted with every injection so very often a sample loop must be changed manually when necessary, limiting the flexibility of the system.
The ACQUITY UPC2 Sample Manager has a novel dual-injection valve design (Figure 14). This
vents the primary sample loop to waste, enabling the sample to enter the loop under atmospheric pressure while maintaining the homogeneity of the mobile phase. In addition, the auxiliary injection valve was designed to reduce pressure pulses from the injection sequence and mitigate carryover, enabling repeatable and reproducible partial loop injections (Figure 15). Injections of 0.1 to 50 μL can be made in 0.1 μL increments, and with dual needle wash options, sample carryover is negligible. Figure 16 demonstrates injection linearity with partial-loop injection from 1 to 10 mL with 1 mL increments.
Optical Detection
Optical detection is sometimes troublesome with analytical SFC systems. Detector flow cells designed for HPLC systems may lead to unacceptable dispersion volume and baseline noise. Refractive index detectors when used for SFC, cause significant baseline noise and curvature with a compressible fluid, amplifying the noise produced by the pumping system. Solvents such as methanol and water, which are commonly used in RPLC, have very similar RI values (Figure 17), and so RI effects in reversed-phase methods are typically not that significant. CO2 has a value that is very different from methanol (the most commonly used co-solvent) making the range of refractive indices of substances larger than in LC increasing baseline noise and limiting sensitivity. As a further challenge, the density, and therefore refractive index, of a CO2-based mobile phase changes over the course of a gradient analysis.
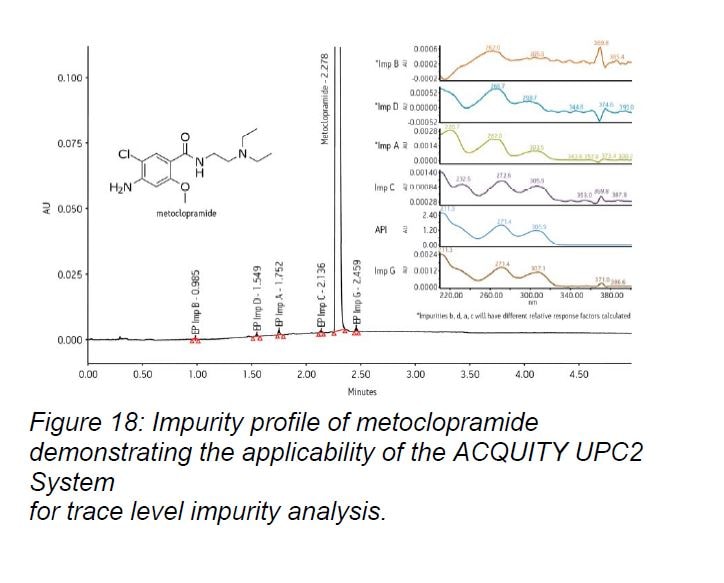
The ACQUITY UPC2 PDA Detector is specifically designed for compressible fluids. Instead of sapphire lenses, which reduce energy throughput at lower UV wavelengths, the ACQUITY UPC2 PDA Detector’s lenses are made of high-strength silica which withstands the back pressure generated during a separation. This helps to maximize sensitivity, reduce baseline noise, and compensate for differences in RI effects between CO2 and the organic co-solvent. The optics bench is thermally controlled to further improve baseline stability and mitigate RI effects. A low-dispersion, stainless steel flow cell accommodates narrow peak widths, while the 10 mm path length maximizes sensitivity and at the same time maintains optimal spectral performance. The exceptional level of sensitivity attainable enables the quantitation of trace level impurities (Figure 18).
Mass Spec (MS) Detection
Akin to the unique instrument requirements for adapting optical detection for the compressible mobile phase of CC, interfacing CC to MS requires modifications to accommodate the compressibility of the mobile phase. The CC-MS interface must allow for the mobile phase to decompress from the pressurized state to atmospheric pressure within the ion source of today’s mass spectrometers. Without careful consideration of the mobile phase compressibility, analyte transport into the ion source can be negatively affected. Poor analyte transport can result in poor peak shape and/or poor ionization. In the worst-case scenario, no ionization will occur and the analyte of interest will not be detected by the mass spectrometer.
The decompression of the compressed mobile phase must be controlled independently of mobile phase flow rate, mobile phase composition, and the post-column system pressure established by the automated back pressure regulator (ABPR). Further, the decompression must be managed without sacrificing efficient analyte transport into the ion source. To achieve these objectives, the ACQUITY UPC2 mass spectrometry interface was designed for a compressible mobile phase and employs a split-flow interface with a makeup fluid. The mass spectrometry interface introduces a constant flow rate of mobile phase, usually between 300 and 500 µL /min (compressed), through the split restrictor to the mass spectrometer. The remaining portion of the mobile phase is directed to the ABPR for control of the post-column system pressure over a wide range of mobile phase flow rates and compositions. A schematic representation of the ACQUITY UPC2 mass spectrometry interface is shown in Figure 19 which highlights the split interface and addition of makeup fluid.
Figure 19: Schematic representation of the ACQUITY UPC2 split-flow mass spectrometry interface.
The role of the makeup fluid in the CC-MS interface is multi-fold. Primarily, it is required for electrospray ionization (ESI) operation under about 5% modifier. Since ESI is a liquid-phase ionization technique, some amount of liquid is required for ionization. As such, when the mobile phase modifier percentage is very low, the amount of liquid present in the mobile phase is insufficient for ESI. Therefore, the addition of liquid in the form of makeup flow is required for ESI at low modifier percentages. Secondly, the makeup fluid can help with analyte transport. At some point along the split restrictor, the CO2 will transition from the high-pressure dense state into the gas state and lose its solvating power. Accordingly, after the CO2 has transitioned into a gas, only the liquid modifier is available to dissolve analytes and transport them into the ion source. When zero or very low percentages of modifier are employed in the separation, no liquid is available to transport analytes through the split restrictor into the ion source for ionization. Makeup fluid is added upstream of the split restrictor to help transport analytes into the ion source under such conditions. An example of peak profiles indicating good and poor analyte transport is shown in Figure 20. In this case, the makeup fluid flow rate was selected for poor analyte transport (15A), and good transport (15B).
Figure 20: Exemplary peak shapes indicating poor analyte transport (A) and good analyte transport (B).
The makeup fluid is also important for transporting analytes when the analyte has limited solubility in the mobile phase liquid modifier. Sometimes the analyte is highly soluble in the mixture of liquid modifier and compressed carbon dioxide but less soluble in the liquid modifier alone. In these cases, the analyte can precipitate from solution after the CO2 transitions to the gas state in the split restrictor, even when there are high percentages of liquid modifier present. When analyte solubility in the liquid modifier is insufficient, the result is poor peak shape, plugging of the interface tubing, and/or poor peak reproducibility. The addition of an appropriate makeup fluid can help avoid such difficulties by increasing the solubility of the analyte in the newly formed mixture of liquid modifier and makeup fluid. For example, highly lipophilic analytes may be highly soluble in a CO2/methanol mobile phase and relatively insoluble in methanol alone. In this case, a non-polar makeup fluid can be added to reduce the net polarity of the liquid modifier and makeup fluid mixture. The lipophilic analyte will be more soluble in the low-polarity liquid mixture and so more readily transported into the ion source.
When necessary, makeup fluids in the CC-MS interface also introduce ionization-enhancing compounds to the mass spectrometer. These compounds can be added after the column, without affecting the separation. Ionizing-enhancing compounds such as 5% (by volume) water and/or 20 mM of one of ammonium hydroxide, formic acid, or ammonium acetate can often increase ionization efficiency in ESI. The concentration and type of ionization-enhancing compound, is very much analyte-specific and should be tuned to each application for optimal response.
Once the composition of the makeup fluid is selected, the flow rate of the makeup fluid can also be tuned for optimal response. A range of flow rates can be screened against the MS response. Too low of a flow rate may result in poor transport while too high of a makeup flow rate often results in reduced MS signal. Along with makeup fluid composition, the optimal makeup fluid flow rate is analyte and method-specific and, if a maximum signal response is desired, they should be optimized with each new application. Further, the makeup fluid composition and flow rate should be re-optimized when switching between ionization techniques, such as, for example, when switching from ESI to atmospheric pressure chemical ionization (APCI).
In summary, the ACQUITY UPC2 mass spectrometry interface has been designed specifically for a compressible mobile phase and for interfacing with a modern mass spectrometer employing atmospheric pressure ionization techniques such as ESI, APCI, ESCi® multi-mode ionization, atmospheric pressure photoionization (APPI), and UniSpray™.
Back Pressure Regulation
One of the most critical parts of any system, handling compressible solvents, is the ability to accurately control and maintain the pressure inside the system. As we’ve seen, imprecise control of back pressure can greatly affect mobile phase density, and therefore analyte solvation and retention times. Traditional SFC systems often suffer from inaccurate and imprecise control of the backpressure due to multiple factors such as: poor pressure monitoring at the back pressure regulator (ABPR), slow-to-respond feedback loops, low-resolution stepper motors, poor control of pressure and flow at the pump, and degradation of ABPR components over time.
The ACQUITY UPC2 System exhibits improved back pressure control through an innovative dual-stage active and static BPR (Figure 21). Through this combination of active and static back pressure control, the static BPR keeps the system at a minimum pressure while the active BPR enhances the control of the set-point defined by the user (Figure 22). In an effort to further improve robustness, the static cartridge BPR is heated to mitigate freezing of the mobile phase that can occur with fast decompression at the ABPR outlet. The dual stage BPR resides within the ACQUITY UPC2 Convergence Manager (CM) (Figure 23). This module also houses the inline particulate filter for the incoming CO2, CO2 leak detector, vent valve, pressure relief valve, and auxiliary injection valve.
Overall System Performance
Finally, the ACQUITY UPC2 System has inherently low dispersion, like the ACQUITY UPLC systems, enabling the use of smaller I.D. and smaller particle size columns (Figure 24). Narrow I.D. columns increase sensitivity, conserve solvent, and utilize flow rates more amenable to mass spectrometry. Smaller particle size columns increase separation efficiency and improve resolution.
Figure 24: Comparison of 5-μm and 1.7μm columns at the same flow rate and column dimensions. By reducing particle size from 5 to 1.7 μm, efficiency was improved threefold, and sensitivity and resolution nearly doubled.