INTRODUCTION TO PREPARATIVE LIQUID CHROMATOGRAPHY (LC)
Chromatography is defined as the separation of a mixture based upon the chemical attributes of the components within that mixture. Preparative (Prep) or purification chromatography is defined as the process of using chromatography to isolate a compound in a quantity and at a purity level sufficient for further experiments or processes. A scientist determines a compound of interest, then develops a method to successfully separate and isolate that compound from starting materials, side reactions or other impurities. The overall goal is to meet increasing needs for high throughput and productivity, while at the same time adapting purification techniques to satisfy appropriate scale, purity and reproducibility requirements.
Purification chromatography is utilized in many scientific settings from large pharmaceutical organizations to small natural product research groups. Although application areas may differ, the general user requirements are quite similar, with a desired final purity level at 95% or greater for the target isolation.
The intention of this primer is to provide liquid chromatographers with introductory information regarding the rapidly expanding field of LC purification. Fundamental principles such as the development of a chromatographic separation method, mathematical equations required for method scale-up, and commonly used modes for fraction collection are introduced. Reversed-phase is the primary mode of separation discussed since it is used in most preparative LC applications.
PURIFICATION BY LIQUID CHROMATOGRAPHY
The set-up for an LC purification system is identical to that of a general liquid chromatographic system, with the addition of a fraction collector. The sample mixture is injected onto a chromatographic column and the components are separated based upon specific chemical or physical properties. As the components are detected, they can be either diverted to waste or collected for further experimentation. The process of collecting the eluate can be as simple as an analyst that manually collects components as they elute, or fully automated, where the detector signals a fraction collector to divert the flow to collection vessels. Purity of the isolated fraction diverted to the collection vessels depends upon resolution of that compound from other close eluting impurities in the separation.
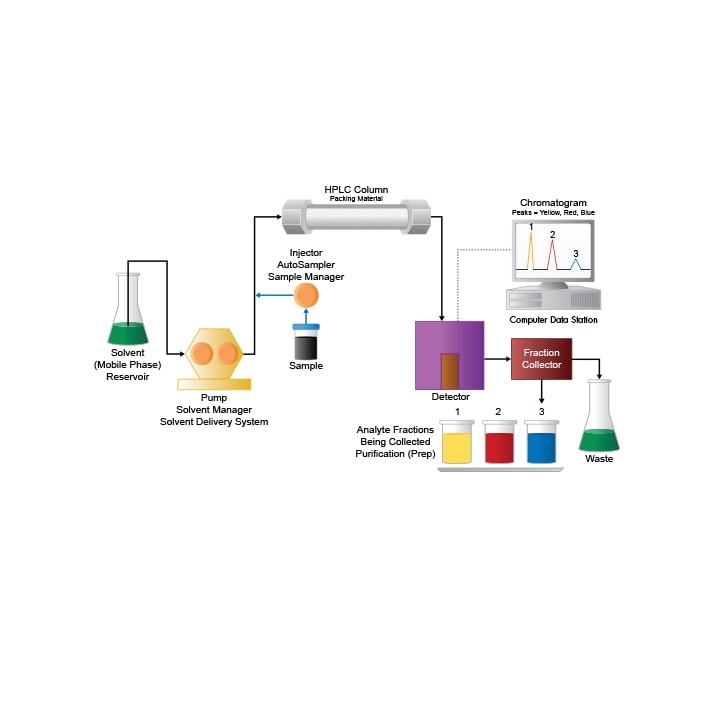
Figure 1: The preparative LC system.
The success of a purification isolation is determined by the throughput, recovery and purity of the isolate obtained. The isolation is driven by peak separation or “resolution.” The following chromatographic parameters have the most significant impact on resolution:
■Column packing material (stationary phase)
■Solvent strength
Conditions that provide optimum resolution are determined by performing a method development workflow. The workflow is quite simple but does consist of multiple steps, ranging in complexity and necessity based on the unique properties of each sample. No matter the application or the mode of sample separation (i.e. reversed- phase, ion-exchange, etc.), the workflow will generally be the same.
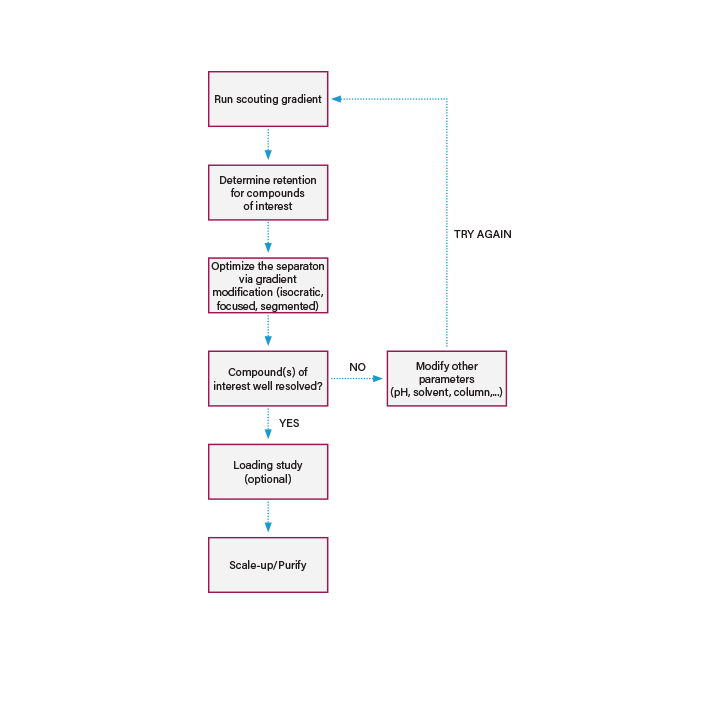
Figure 2: General preparative workflow.
The most important factor to be considered before designing a workflow is the nature of the target compound. If isolating a known compound from the same or a new source, it is easy to obtain literature information on the chromatographic behavior of the target compound and the appropriate method for isolation can be selected from previously published methods with little difficulty. However, it is more difficult to design an isolation protocol for a crude extract where types of compounds are unknown. In this case, a series of exploratory experiments can be carried out after the initial isolation to learn more about the compound of interest such as pKa, molecular weight, solubility, stability, UV spectra and biological activity. From this information, the initial separation method can be modified to meet the chemical and physical requirements of the compound. Not only is this information helpful in method development, it is valuable post collection when stability of the isolated product becomes important.
The first step is to prepare a sample and perform a general separation using a rapid scouting gradient. This is typically executed at small scale to conserve precious sample material. From this separation, the solvent condition that elutes the compound of interest can be calculated and the separation conditions further optimized to maximize resolution. A loading study may also be performed to determine how much sample can be loaded while maintaining a resolution adequate for producing a highly pure isolate.
After the separation method and desired sample load have been established, method scale-up is often, though not always performed for any isolate with promise or potential value. The term "scale" for the purposes of this primer is intended to describe the instrumentation and methodology required to meet the purity, throughput and yield goals for the application. When a method is “scaled-up” it is moved to a system with hardware suited to handle higher sample loads. Scale-up essentially involves moving from an analytical to prep diameter column while maintaining resolution through a series of scale-up calculations and hardware changes.

Figure 3: Comparison of column internal diameters used for analytical and prep scale purification.
SAMPLES
Samples from which a particular compound can be isolated come from a wide range of sources including drug intermediates, natural products, nutraceuticals, beverages or industrial products. As long as a material can be extracted and dissolved in a liquid matrix, the individual components can be purified using chromatography.
Techniques used for sample extraction depend on the complexity of the material from which is extracted. For natural products, the sample is typically dried and ground into fine particles to increase the efficiency of the extraction. Then a technique such as percolation, for large sample sizes, or maceration, for small sample sizes, can be used to extract the compounds of interest. Both techniques involve adding a solvent to the sample material and collecting the solute after sonication, swirling or soaking. After the extract is collected, as with all HPLC samples, it must be filtered to remove particulates and free from bubbles prior to injection.
MODES OF SEPARATION
There are four primary modes of separation used in preparative chromatography. These include reversed-phase, normal phase, gel permeation and ion exchange.The appropriate separation mode is determined by the compatibility of the stationary phase and solvents with the sample, extract or mixture being analyzed.
Reversed-phase, the most common technique used for purification development uses a stationary phase that is less polar than the eluting solvent. The eluent is a mixture of water and acetonitrile or methanol, while buffers (acids or bases) are added to control the degree of sample ionization and occupy free unreacted silanol groups present in the stationary phase. The bonded phase materials or sorbents in silica-based stationary phases are derivatized with alkylsilyl reagents to reduce peak tailing and improve chromatographic reproducibility. The degree of carbon loading with these derivatized reagents (silianization) can give columns made by different manufacturers, unique separation characteristics.¹
QUICK START METHOD DEVELOPMENT
To establish a reversed phase separation, often a small scale analytical column (≤ 4.6mm diameter) is selected with a length and packing material available in large scale (≥ 10mm diameter) diameter to facilitate future scale-up. Finding the correct solvent system to achieve optimal separation is key. For acidic compounds, generally an aqueous mobile phase is prepared at an acidic pH, while either methanol or acetonitrile is prepared as the strong solvent. Formic acid, equal to 0.1% by concentration, is a commonly used buffer additive because it is both UV and mass spectrometry compatible. MS compatibility is helpful when identifying unknown compounds and preparing a purified isolate for further study. A basic pH mobile phase can be used rather than acidic pH, to maintain the unionized form of basic molecules. The column stationary phase package insert should be consulted for compatibility before using a buffer at any pH.
The aqueous mobile phase is typically placed in pump position A and the strong solvent placed in pump position B. “A” and “B” will be used throughout this primer to refer to aqueous and strong solvent in the pump tables, respectively.
|
Next >
|