A Powerful Tool for Improved Sample Preparation
As an analytical scientist, you are faced with many challenges when determining what tools you can best use to achieve the desired result. Determining which sample preparation tools and approaches are important considerations that can significantly impact your success.
Ideally, you would be happy if you did not have to do any sample preparation. In reality, however, sample preparation is often necessary. You may need to optimize a method for an existing sample to improve throughput or lower the cost per analysis. Or, you may be asked to analyze a wide variety of different types of samples to report on new compounds of interest. Each new sample type can present different analytical challenges. In addition, scientists today are faced with the significant challenge of reporting values at lower concentration levels than ever before, without compromising accuracy and precision.
This book is designed to help you explore and understand a very powerful tool in sample preparation technology: solid-phase extraction [SPE]. You will see how this technology, which uses devices with chromatographic packing material, can help meet your analytical challenges.
Definiton of Solid-Phase Extraction
SPE is a sample preparation technology that uses solid particle, chromatographic packing material, usually contained in a cartridge type device, to chemically separate the different components of a sample. Samples are nearly always in the liquid state [although specialty applications may be run with some samples in the gas phase]. Figure 1 shows a sample, which appears black, being processed on a SPE device so that the individual dye compounds, which make up the sample, are chromatographically separated.
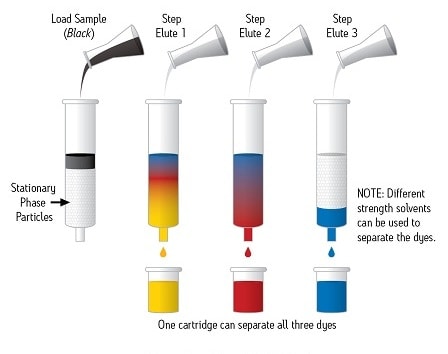
Figure 1: Examples of an SPE Method
The chromatographic bed can be used to separate the different compounds in a sample, to make subsequent analytical testing more successful. For example, SPE is often used for the selective removal of interferences.
The technically correct name for this technology is “Liquid-Solid Phase Extraction,” since the chromatographic particles are solid and the sample is in the liquid state. The same basic chromatographic principles of liquid chromatography that are used in HPLC are also used here, but in a different format and for a different reason. Here, chromatography is used to better prepare a sample before it is submitted for analytical testing.
In sample preparation, samples can come from a wide range of sources. They can be biological fluids such as plasma, saliva, or urine; environmental samples such as water, air, or soil; food products such as grains, meat, and seafood; pharmaceuticals; nutraceuticals; beverages; or industrial products. Even mosquito heads can be the sample! When a scientist needed to analyze neuropeptides extracted from the brains of mosquitoes, SPE was the sample preparation method of choice [Waters Applications Database, 1983].
Four Major Benefits of SPE
There are many benefits to using SPE, but four major benefits deserve special attention.
1. Simplification of Complex Sample Matrix along with Compound Purification
One of the most difficult problems for an analytical chemist is when compounds of interest are contained in a complex sample matrix, such as mycotoxins in grains, antibiotic residues in shrimp, or drug metabolites in plasma, serum, or urine. T he large number of interfering constituents or substances in the sample matrix along with the compounds of interest makes analysis extremely difficult.
The first problem to solve is the resulting complexity of the analysis itself due to the presence of so many entities which must be separated in order to identify and quantitate the compound[s] of interest. See Figure 2.
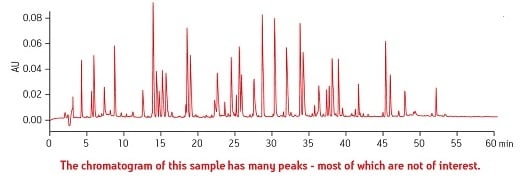
Figure 2: Example of a Complex Sample
The robustness of the assay may not be adequate, because any slight change could impact the resolution of the separation of a critical pair of analytes.
Another consideration is that the presence of all the interferences in the original sample matrix can result in instrument downtime due to a buildup of contamination with each injection. If the interferences were removed as part of the sample preparation, then the compounds of interest could be analyzed with a simpler, more robust method. This can be seen in Figure 3, which compares the original sample on top to the new SPE-prepared sample on the bottom.
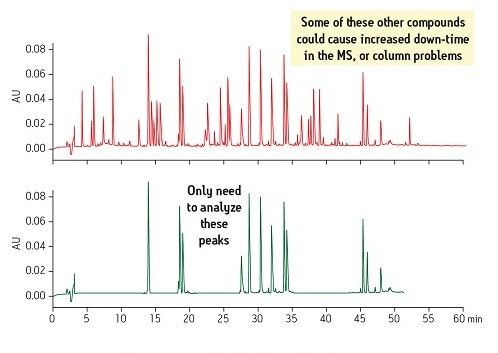
Figure 3: Comparison of Sample Matrix Complexities
An additional benefit of simplifying the sample matrix is improved quantitation accuracy. T he top blue trace for compound 1 in Figure 4, initially appears to be acceptable. However, it really has some contamination from the sample matrix when compared to the blank sample matrix trace in red shown just beneath it. With a proper SPE protocol, the lower traces show the same compounds with no problems with interference, making quantitation much more accurate.
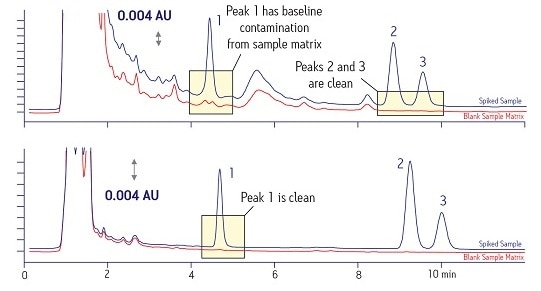
Figure 4: Improved Quantitation with Better Sample Preparation
Another example is shown in Figure 5. T he upper trace shows significant interference from the sample matrix on both compounds 1 and 2. T he lower trace shows much improved results [clean base line] due to proper sample preparation with SPE. Notice a much cleaner baseline improves the accuracy of the analytical results. Also, a much purer extract can be obtained if the sample requires isolation and purification of that compound.
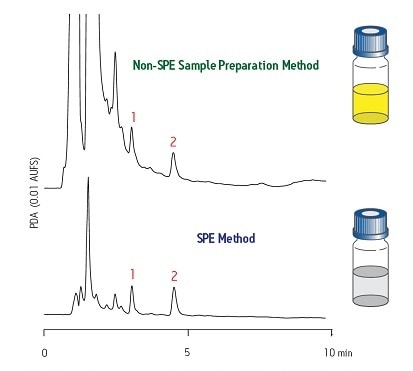
Figure 5: Significant Improvement in Baseline Using SPE Technology
2. Reduce Ion Suppression or Enhancement in MS Applications
The second problem with complex sample matrices can be seen when we look at mass spectrometer output [LC/MS or LC/MS/MS]. For proper MS signal response [sensitivity], the compound ion must be allowed to form properly. In cases where the formation of the compound ion is suppressed by interferences in the sample matrix, the signal strength is greatly diminished.
We can see this effect in Figure 6. The upper output is the signal for our compounds of interest when injected in a saline solution. The lower trace shows significant reduction in response [> 90% suppression] of these same compounds when they were analyzed in human plasma. For the lower trace, only a common protein precipitation step was performed. This technique does not clean up the matrix interferences that cause the ion suppression, resulting in poor signal response.
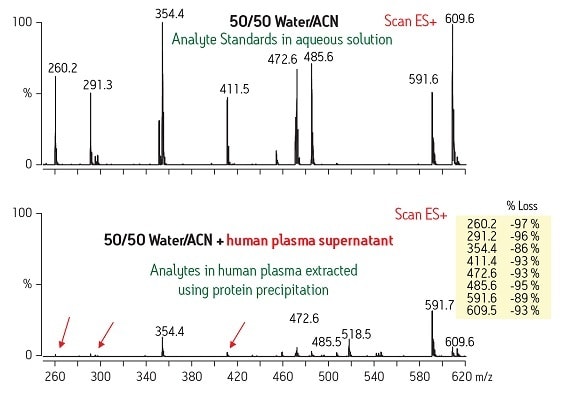
Figure 6: Example of Ion Suppression Due to Sample Matrix
Another good example of this suppression effect can be seen in Figure 7. In the upper trace of the MS output, where the plasma sample was prepared with just a protein precipitation step, we can see that the terfenadine peak is suppressed by 80%. In the lower trace, where the same sample was prepared with a SPE method, we can see minimal ion suppression. Because the interferences from the sample matrix were removed, this allowed the compound ion to form properly, creating a better signal.
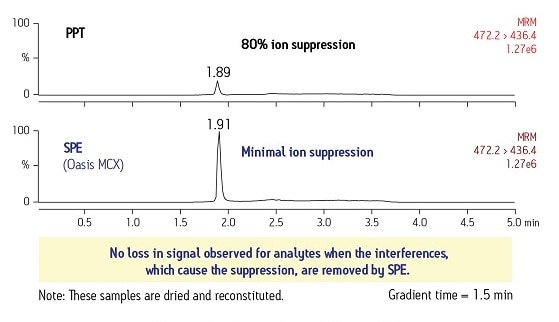
Figure 7: Reduced Ion Suppression with Proper SPE
In some instances, interferences from the sample matrix can artificially increase the signal reported for a compound. This is called ion enhancement, resulting in an inaccurately high reported value. A proper SPE method will minimize this effect by cleaning away the interferences from the compound, resulting in a more accurate reported value.
3. Capability to Fractionate Sample Matrix to Analyze Compounds by Class
An analyst may be faced with a sample that contains many compounds, with a need to separate them by class so that further analysis can be carried out much more efficiently. For example, a soft drink beverage contains a wide range of compounds in its formulation. An SPE method could be developed to separate the different classes of compounds, for example by their polarity. The polar compounds could be collected, as a separated fraction, from the more non-polar compounds. These two fractions could then be separately analyzed in a much more efficient way because their compounds would be more similar.
An example of the power of fractionation by SPE is shown in Figure 8. Here, a complex sample of a dry powder [purple grape drink mix] is easily separated into four fractions: a fraction of just the polar compounds, a purified red compound, a purified blue compound, and a fraction containing all the remaining very non-polar compounds. You will see elsewhere in this book how very powerful this capability can be.
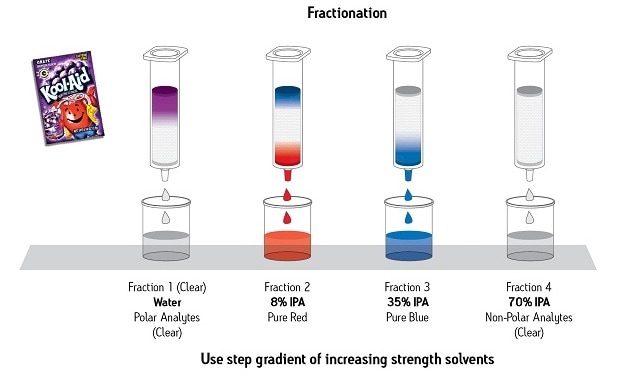
Figure 8: Sample Preparation by SPE
For a more detailed discussion of sample fraction by SPE see page 125 in the Method Development section.
4. Trace Concentration [Enrichment] of Very Low Level Compounds
Analysts today often need to report on compounds at far lower concentration levels than ever before, as little as parts per trillion [ppt] and even lower. Typically these levels are lower in the neat sample than the sensitivity capability of the analytical instruments.
A good example of this is the analysis for trace contaminants in environmental samples or metabolite development over time in biological fluids. The upper trace in Figure 9 shows the poor response of the original neat sample for the compound of interest. Using the same analytical conditions but with the sample prepared with SPE used in a trace concentration strategy, the lower trace shows a dramatic increase in signal strength for this compound. With this result, an accurate calculation of the original compound concentration in the neat sample can be made.
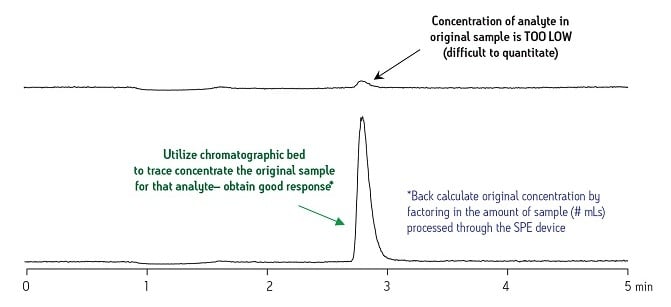
Figure 9: Example of Trace Concentration
Without the retention capability of chromatographic packing materials in SPE, the ability to trace concentrate a specific compound[s] would be very difficult, if not impossible, with other sample preparation approaches.
Summary
As we’ve seen, an SPE device with a chromatographic bed can perform four critical functions to make the analysis of the sample more successful. See Figure 10.
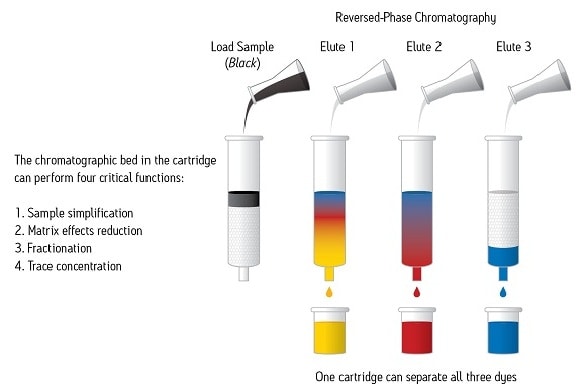
Figure 10: The Power of SPE
In this book, we have endeavored to provide all of the SPE fundamentals and success techniques derived from scientists from all over the world who have counted on this technology in the past thirty years. Today, scientists are finding SPE more useful than ever in solving difficult sample preparation and analytical problems.
We hope this book will enable you to understand and master the capabilities of SPE, so that you too can put the power of this technology to use in your laboratory.