The BioAccord LC-MS System comes installed with pre-optimized biopharmaceutical workflows for:
Each workflow provides for compliance-ready data acquisition, processing and reporting with optimized default settings, automated data processing and reporting capabilities, and full audit trail capabilities.
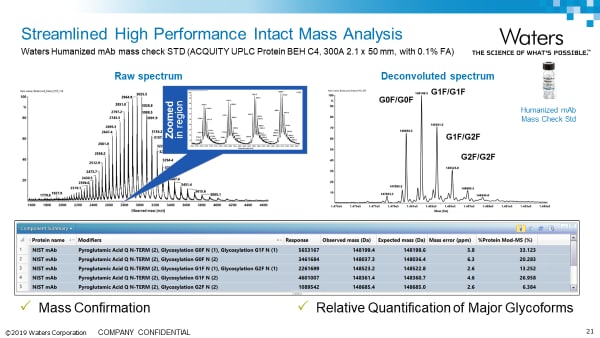
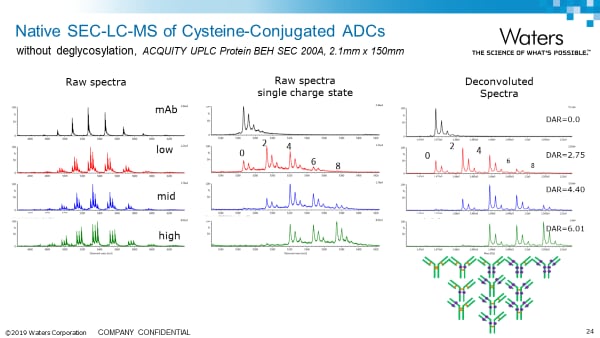
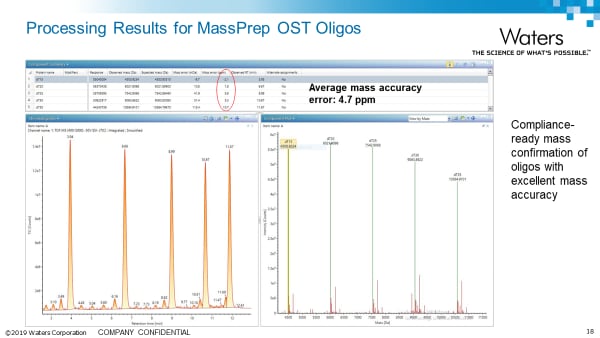
Intact Analysis Application notes:
Analysis of Antibody Drug Conjugates (ADCs) by Native Mass Spectrometry on the BioAccord System
Enabling Routine and Reproducible Intact Mass Analysis When Data Integrity Matters
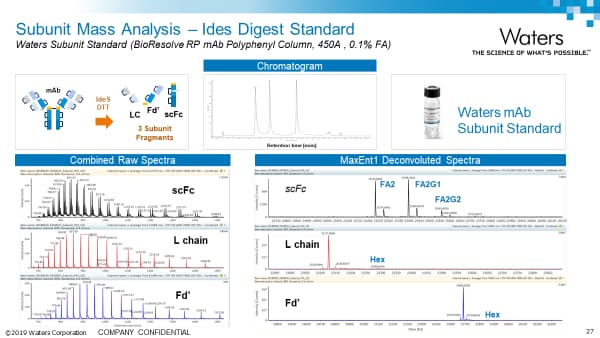
mAb Subunit Analysis Application Notes:
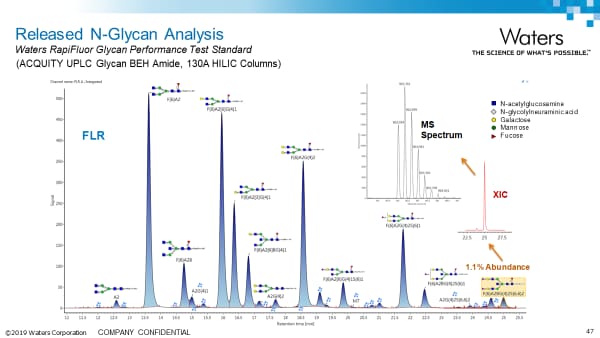
Released N-Glycan Analysis Application Notes:
Released N-linked Glycan Analysis Using the BioAccord System
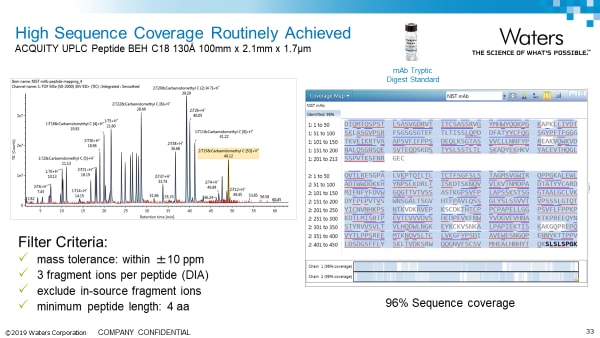
Peptide Mapping/MAM Application Notes: