Addressing System Pressure
In the previous section, the benefit of smaller chromatographic particles and minimized system band spreading [both instrument and column] was established. UPLC Technology facilitates improved chromatographic performance by minimizing system band spreading to produce more efficient separations in less time, thus achieving better data quality. Band spreading, however, is not the only factor that dictates the performance one can achieve with small particles. The available pressure of the instrument also plays a large role.
Pressure is inherently generated when mobile phase passes through the connective tubing from the pump to the injector, the injector to the column, the column itself, the tubing post column as well as the detector cell. The measurement of system pressure is a cumulative effect of all of these components [instrument and column]. As flow rate is increased, the pressure produced by mobile phase flowing through the connective tubing itself will increase. Additionally, the ID of the tubing, as well as its length, will also impact how much pressure will be generated in combination with the flow rate. The pressure difference between two columns can be compared against theoretical predictions if the pressure generated by the instrument itself, is subtracted from the total system pressure [instrument + column].
As particle size is reduced, back pressure increases at a rate that is inversely proportional to the square of the particle diameter. Simultaneously, the optimal mobile-phase speed [linear velocity] increases with decreasing particle diameter. Therefore, the pressure at the optimal linear velocity for a given particle size increases at a rate that is inversely proportional to the cube of the particle diameter [Figure 44].
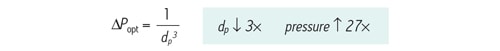
Figure 44: The relationship between optimal pressure [∆Popt] and particle size [dp] for a constant column length. If particle size is decreased by a factor of 3, pressure will increase 27´.
This is a significant limitation when trying to utilize smaller particle columns on conventional HPLC instrumentation to improve chromatographic resolution [keeping column length constant while reducing particle size] or to improve the speed of analysis while maintaining resolution [keeping the L/dp ratio constant]. Due to pressure limitations of conventional HPLC instruments
[350–400 bar; 5000–6000 psi], the use of smaller particles often results in a restriction of column length or operating at sub-optimal linear velocities [flow rates].
For a constant column length, theory predicts if particle size is decreased from 5.0 µm to 1.7 µm [3´ decrease in particle size], backpressure is anticipated to increase 27´. Matching close to theoretical predictions, system pressure increased 22´ when transitioning from a 5.0 µm column to a 1.7 µm column of the same length. As observed, the 1.7 µm column is operating well above the upper pressure limit of a conventional HPLC instrument [Figure 45].
The large increase in backpressure observed by decreasing particle sizes is one of the primary reasons why sub-2 µm particle columns [and corresponding LC instruments] were never commercially successful until the advent of the ACQUITY UPLC System.
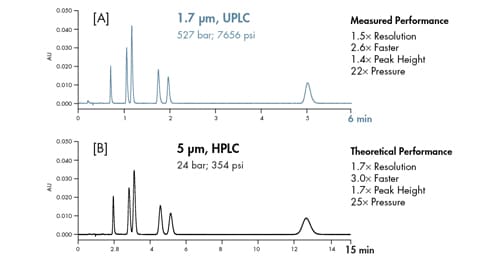
Figure 45: Influence of particle size and optimal flow rate on column pressure [subtracted from total system pressure]. Constant column length. 2.1 x 50 mm columns; flow rate = 0.6 mL/min [1.7 µm] and 0.2 mL/min [5 µm].
If the separation goal is to maintain resolution while reducing analysis time [keeping the L/dp ratio constant], the increase in pressure is much less than holding column length constant while reducing particle size. The change in pressure is inversely proportional to the square of the particle size [rather than the particle size cubed] due to the proportional reduction in column length.
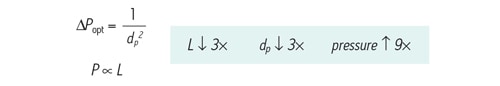
Figure 46: The relationship between optimal pressure [∆Popt] and particle size [dp] for differing column length. If particle size and column length are decreased by a factor of 3, pressure will increase 9´.´ faster].
In this example, both column length and particle size are reduced 3´ [Figure 47]. This means backpressure is anticipated to increase 9´. The observed values match closely with theoretical predictions. Keeping L/dp constant, an 11´ increase in back pressure is observed when transitioning from a 5.0 µm, 150 mm long column to a 1.7 µm, 50 mm long column.
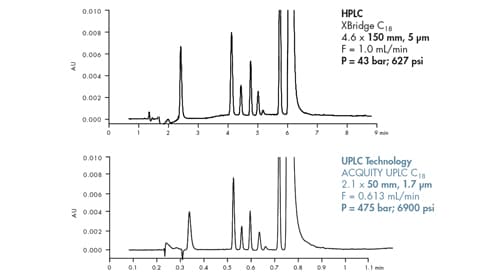
Figure 47: Influence of particle size, column length and optimal flow rate on column pressure [subtracted from total system pressure]. Constant L/dp ratio. Note the significant difference in analysis time [the UPLC separation is 7
When run at their optimal flow rate, the pressure produced by smaller particles will exceed the pressure limitations of conventional HPLC systems. The ACQUITY UPLC System [upper pressure limit of 1030 bar, 15,000 psi] was designed to accommodate these pressures, enabling sub-2 µm particles to be successfully run at their optimal flow rate.
Elevated Temperature
One approach towards compensating for the higher pressures produced by small particles is to elevate the column temperature. As column temperature is increased, mobile-phase viscosity decreases, resulting in lower backpressure [if flow rate is held constant]. However, the speed at which analyte molecules move in and out of the stationary phase pores [diffusion] also increases, resulting in the need to increase flow rate in order to maintain performance.
When increasing column temperature from 30 °C to 90 °C, flow rate must be increased in order to maintain efficiency [Figure 48]. No gain in efficiency is observed when comparing the highest plate count at either temperature which is in agreement with chromatographic theory.
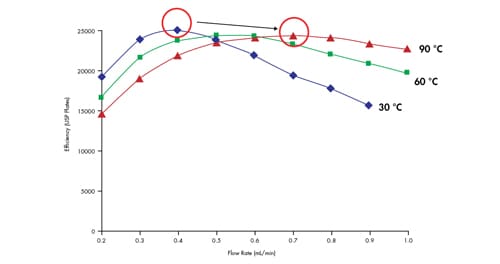
Figure 48: The effect of column temperature on efficiency. Isocratic retention of amylbenzene on an ACQUITY UPLC BEH C18 2.1 x 100 mm, 1.7 µm column.
A more interesting comparison can be made if plate count is plotted against system pressure [Figure 49]. By plotting the data in this way, one can clearly see that the maximum column efficiency is achieved at approximately the same system pressure, independent of the temperature of the separation. This means that elevated temperature cannot be used to circumvent the pressures associated with the use of small particles. In other words, a conventional HPLC instrument is not suitable for the efficient use of very small particles.
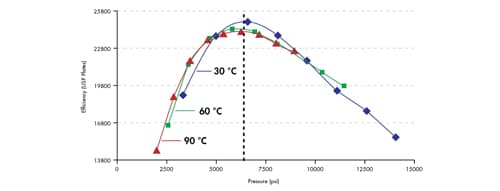
Figure 49: Maximum efficiency is achieved at similar pressures, independent of temperature. Isocratic retention of amylbenzene on an ACQUITY UPLC BEH C18 2.1 x 100 mm, 1.7 µm column.
| < Previous |
Next >
|