Peptides and proteins consist of many amino acids that are bound together via peptide bonds. These biomolecules in turn make up many different samples, such as biotherapeutics, foods, and feeds. To analyze the amino acids contained within these biomolecules, it is critical that the bonds be hydrolyzed to form free amino acids. However, during this process the different chemical properties of the conjugated amino acid bonds can affect both the efficiency of the cleavage of the amino acid bonds and the recovery of the individual amino acids. For example, the recovery of the amino acids during hydrolysis can be impacted by specific chemical reactions, reagents matrix interferences, and the stability of the amino acids themselves.
Given the wide differences in the chemical and physical properties of samples and amino acids, different hydrolysis procedures have been developed over the years. The procedures vary by the type of reaction (chemical or enzymatic), the nature of the chemical reaction (acid or base), and the physical state of the reaction (liquid or vapor). The differences can impact the recovery of specific amino acids – which may be destroyed by specific reagents – or the efficiency and time required for the hydrolysis. In some cases, multiple hydrolysis procedures may be needed to determine the total amino acid content of a sample. The common types of chemical hydrolysis reactions and the modes of hydrolysis are described below.
Note: Enzymatic hydrolysis, an uncommonly used procedure, is not covered in this document.
Also note that the many chemical hydrolysis reactions can be performed with different types of equipment. Historically, hydrolysis procedures used a heat source under vacuum to ensure completion of the reaction, but with more modern equipment, microwave-induced hydrolysis has also become widely used. The benefits of each method differ, and you should investigate them prior to equipment selection.
Acid hydrolysis is the most common method for hydrolyzing a protein sample, and the method can be performed in either vapor or liquid phase. Although a range of different acids can be used for this reaction, the most common is 6 M HCl. Because HCl is evaporative, it can also be used to recover the hydrolysate in smaller amounts of buffer, a feature that is particularly useful for small amounts of sample. Furthermore, the versatility of HCl allows it to be used in either liquid- or vapor-phase hydrolysis.
The acid-hydrolysis reaction with 6 M HCl results in the addition of water to each covalent peptide bond, yielding the desired individual amino acids (Figure 1). However, not all amino acids are completely recovered under hydrolysis by HCl. Some amino acids are hydrolyzed to their acid forms, such as asparagine and glutamine, which form aspartic acid and glutamic acid, respectively. In addition, other amino acids cannot be reliably measured. For example, tryptophan is destroyed during the reaction, while sulfur-containing amino acids (e.g., cysteine, methionine) cannot be reliably measured due to partial destruction of the amino acids. Furthermore, amino acids such as tyrosine, serine, and threonine may have lower recoveries due to the nature of acid hydrolysis.
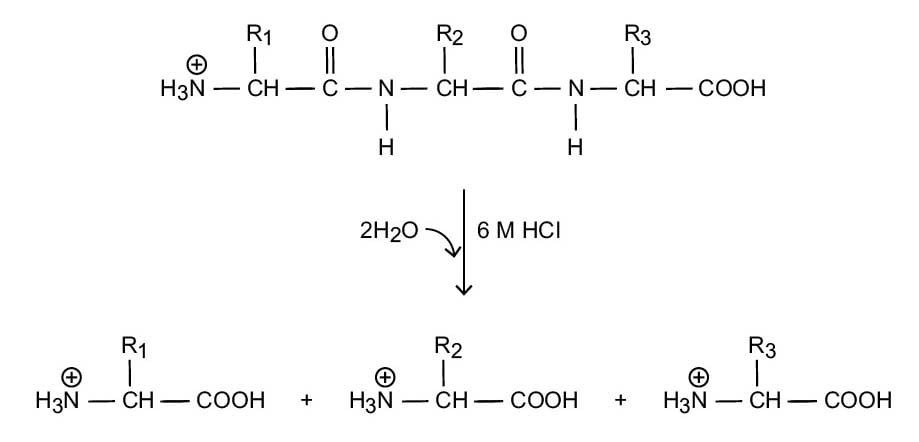
Figure 1. Acid hydrolysis of protein.
However, some of the sulfur amino acids (e.g., cysteine, methionine) can be preserved in acid hydrolysis by HCl if a pretreatment is performed. To accurately quantify these amino acids, the sample can be oxidized or alkylated prior to acid hydrolysis by HCl. For oxidation, typically with performic acid, the sulfur-containing amino acids are oxidized prior to acid hydrolysis by HCl. This results in accurate quantitation of these amino acids in their oxidized forms. Alkylation alternatively allows for preservation of the sulfur- containing amino acids (cysteine) for accurate quantitation in their alkylated forms. Two of the most common alkylating reagents produce cysteine in either of two forms, pyridylethyl cysteine or carboxymethyl cysteine. An added advantage of this alkylation process is that it does not impact other amino acids.
Lastly, given the challenges quantifying some amino acids by HCl hydrolysis, there are alternative acid hydrolysis techniques that can be applied for specific amino acids. One technique uses sulfonic acids, such as methanesulfonic acids (MSA) to quantify tryptophan and methionine (in the sulfoxide form). While this reagent is non-volatile, it does preserve tryptophan and methionine sulfoxide for quantitation.
While acid hydrolysis using HCl is by far the most common technique to hydrolyze proteins and peptides, alkaline or base hydrolysis is often used to measure tryptophan. Because tryptophan is stable under basic conditions, this technique gives accurate quantitation of tryptophan and is widely used for a variety of samples, from foods and feeds to peptides and proteins. Alkaline hydrolysis typically uses NaOH or KOH as the reagent. However, alkaline hydrolysis cannot replace acid hydrolysis for the quantitation of all amino acids. Under alkaline conditions, arginine, cysteine, serine, and threonine are destroyed and cannot be quantified. Other amino acids are also affected, so alkaline hydrolysis is typically used only for tryptophan.
Hydrolysate reactions are performed in either liquid or vapor phase. Regardless of the mode, instrumentation designed specifically for hydrolysis facilitates the reaction. Historically, many hydrolysate reactions occurred at high temperatures and under vacuum over a period of hours to days, but with the advent of microwave-hydrolysis instrumentation, the same process can occur over minutes at lower temperatures.
In liquid-phase hydrolysis, the sample and HCl are both added directly to the hydrolysis tube for reaction. This procedure requires adding the sample, the internal standard, and the acid directly to the hydrolysis tube. The tube is flushed with nitrogen and then sealed and heated for the time needed to complete hydrolysis. Liquid hydrolysis can take hours to days to complete. This procedure is typically used for more complex samples.
In vapor-phase hydrolysis, the sample only reacts with HCl in the vapor phase. The procedure requires placing the sample and the internal standard (if used) in hydrolysis tubes. The sample is then dried, and each tube is placed open in a hydrolysis vessel. The acid (6 M HCl) is added to the bottom of a vessel containing the tubes, and the vessel is then sealed and evacuated and flushed with nitrogen. The vessel is heated for the time required to complete hydrolysis. This mode reduces contamination from any impure reagents, such as HCl. Vapor-phase hydrolysis typically occurs at a faster rate than liquid- phase hydrolysis. In vapor-phase hydrolysis, samples typically need to be of high purity.
| < Previous | Next > |