What is Solid-Phase Extraction (SPE)?
Don't be confused by the term solid-phase extraction [SPE]. A typical SPE device has 50 times more separation power than a simple, single liquid-liquid extraction. SPE is actually column liquid-solid chromatography. Since SPE is liquid chromatography [LC], its practice is governed by LC principles. A sample is introduced into a column or a cartridge device containing a bed of appropriate particles, or other form, of a chromatographic packing material [stationary phase]. Solvent [mobile phase] flows through the bed. By choosing an appropriate combination of mobile and stationary phases, sample components may pass directly through the column bed, or they may be selectively retained.
Individual compounds in the sample each typically appear to travel at different speeds through the device. Using a weaker solvent causes them to move slowly and/or be strongly retained. A stronger solvent speeds up their passage through the bed and elutes the analyte(s) in a more concentrated volume. Elution from an SPE device is usually done by increasing the strength of the mobile phase in a series of discrete, rather than continuous, steps during which selected analytes or interferences are either fully retained or rapidly eluted-this variation of gradient elution called a step gradient.
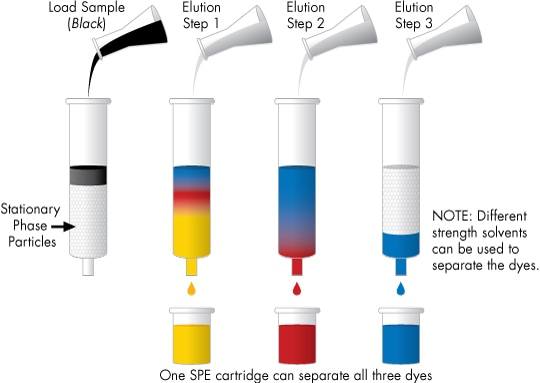
Most commonly, SPE is practiced using miniature column or cartridge devices. An example is shown here. A mixture of three dyes is loaded onto the cartridge in a weak solvent, causing strong sample retention in a narrow band that appears black at the column inlet. Subsequent gradient steps, each with a successively stronger solvent, are used to elute the dyes individually [yellow, red, then blue].
Typical SPE cartridges are low-pressure devices-constructed of solvent-resistant plastic or glass-filled with particles ≥30 µm in diameter. Suitable flow rates may be achieved by gravity or with the assistance of vacuum or low positive pressure. [The latter requires putting a cap on the open inlet of a column or using a sealed device with inlet and outlet fittings.]
Importance of Sample Preparation
In the last two decades, dramatic advances in analytical instrumentation and laboratory information management systems shifted the analyst's predominant tasks from assay measurements to sample preparation and data processing. As the stringency of requirements for higher sensitivity, selectivity, accuracy, precision, and number of samples to be processed has escalated, the corresponding increases in speed and sophistication of analysis and data collection have outpaced improvements in the many traditional techniques of sample collection and preparation. By some estimates, 75 to 80% of the work activity and operating cost in a contemporary analytical lab is spent processing and preparing samples for introduction or injection into an analytical separation and/or measurement device. Clearly, efforts directed and products designed to streamline sample preparation protocols are essential to future progress in analytical science.
Goals of Sample Preparation
Successful sample preparation for most analytical techniques [HPLC, GC, spectrophotometry, RIA, etc.] has a threefold objective: namely, to provide the sample component of interest
To accomplish these goals, a sample, or a representative portion thereof [not always easy to obtain], is prepared via traditional methods of dissolution, homogenization, extraction [liquid- or solid-phase], filtration, concentration, evaporation, separation, chemical derivatization, standardization [internal or external], etc.
Usually such methods are used in combinations of multiple steps, which form a sample prep protocol. The fewer steps and methods used in any given protocol, the simpler, more convenient, cost effective, and less time consuming it is. Simpler protocols lend themselves more readily to automation and also lead to increased accuracy, reliability, reproducibility, and safety.
Innovation in Sample Preparation Methods
There are many ways to combine standard tools and techniques to accomplish the goals of sample prep. However, it is best to seek innovative means to streamline sample prep protocols:
Benefits of Solid-Phase Extraction [SPE] Cartridges
When compared to other sample preparation processes, solid-phase extraction using SPE cartridges offers:
| Lower Cost | • lower solvent consumption • lower reagent consumption • less apparatus |
| Greater Recoveries | • minimal sample transfer |
| Faster Protocol | • fewer steps |
| Greater Safety | • less exposure to toxic agents |
| Greater Accuracy | • no cross contamination |
| No Emulsion Problems | • less sample handling • fewer steps |
| No Transporting of Samples to Lab | • direct field sampling |
| Reduced Harm to Labile Samples | • minimal evaporation |
| Minimal Glass Breakage | • less glassware used, less to wash |
Achieving Sample Preparation Objectives with Solid-Phase Extraction [SPE]
| < Previous | Next > |