
In this application note, an approach is described using TOF screening and a chemometric workflow to compare the similarities and differences between packaging materials, and to identify potential food packaging migrants in infant formula containers.
Packaging has become an indispensible element of food manufacturing processes. Packaging not only better protects consumers from microorganisms, biological, and chemical changes in food, thus providing longer shelf life, but it also makes foods easier to transport.
Recently, food packaging issues have gained widespread importance in food safety, due to the possible migration of chemicals from food contact materials into the food. Instances, such as the leaching of bisphenol-A (BPA) and BPA diglycidyl ether (BADGE) from plastic films to aqueous food simulants,1,2 have caused serious health and legal issues. This incident led to more strict legislation by the European Union3 and the U.S. Food and Drug Administration4 that restricts packaging migration into foods, and better ensures consumer safety.
The internal surfaces of cans used to pack infant formula are often coated with layers of an epoxy liner that forms a barrier between the food and the metal of the can. However, the inert properties of this coating have raised important safety concerns, and thus the possible migration of contaminants from this surface is being actively investigated.
A wide variety of coating materials are used in food packaging, depending on the type of packaging and the food that is contained within the package. In many cases, the particular coating materials used to protect foods are not known to the analyst, thus posing a challenge in identifying potential chemicals that can migrate from the packaging into foodstuffs.
In this application note, an approach is described using TOF screening and a chemometric workflow to compare the similarities and differences between packaging materials, and to identify food packaging migrants in infant formula containers.
|
LC system: |
ACQUITY UPLC |
|
Runtime: |
10 min |
|
Column: |
ACQUITY BEH C18 1.7 μm, 2.1 x 100 mm |
|
Column temp.: |
40 °C |
|
Mobile phase A: |
Water with 0.1% formic acid |
|
Mobile phase B: |
Methanol with 0.1% formic acid |
|
Flow rate: |
0.45 mL/min |
|
Injection volume: |
5.0 μL, PLUNO injection |
UPLC gradients are detailed in Table 1
|
|
Time (min) |
Flow rate (mL/min) |
%A |
%B |
Curve |
|---|---|---|---|---|---|
|
1 |
Initial |
0.45 |
90 |
10 |
0 |
|
2 |
0.25 |
0.45 |
90 |
10 |
6 |
|
3 |
7.75 |
0.45 |
0 |
100 |
6 |
|
4 |
8.5 |
0.45 |
0 |
100 |
6 |
|
5 |
8.51 |
0.45 |
90 |
10 |
6 |
|
6 |
10 |
0.45 |
90 |
10 |
6 |
Table 1. ACQUITY UPLC gradient for a 10-min screening run.
|
MS system: |
Xevo G2 QTof |
|
Ionization mode: |
ESI + |
|
Scan time: |
0.2 s |
|
Capillary voltage: |
2.4 kV |
|
Sampling cone: |
30.0 V |
|
Extraction cone: |
4.0 V |
|
Source temp.: |
150 °C |
|
Desolvation temp.: |
500 °C |
|
Desolvation gas: |
1000 L/hr |
|
Cone gas: |
20 L/hr |
|
Mass range: |
50 to 1000 m/z |
|
MSE conditions |
|
|---|---|
|
Low energy: |
6 eV |
|
High energy ramp: |
20 to 35 eV |
|
Set mass: |
188.08 m/z |
|
Scan time: |
1.0 s |
|
Collision energy: |
25.0 eV |
|
Mass range: |
50 to 500 m/z |
|
Compound: |
Leucine enkephalin |
|
Masses: |
m/z 556.2771 (MSE); m/z 556.2771; and m/z 278.1141 (MS/MS) |
|
Flow rate: |
25 μL/min |
|
Capillary voltage: |
2.7 kV |
|
Collision energy: |
21.0 eV |
The infant formula was purchased from a local supermarket. The contents were emptied and the container was washed and dried with nitrogen gas.
The tin was heated to 110 °C for 5 min to promote packaging migrants onto the surface of the tin, and 100 mL of methanol/water (50:50) was added into the tin. An aliquot of 2 mL (Day 0) was removed and stored in a -80 °C freezer. The remaining solvent in the tin was incubated at 40 °C. An aliquot of 2 mL was collected at the following time points, Days 1, 2, 3, 4, 5, 6, 7, and 8; and stored at -80 °C until analysis.
The samples were analyzed according to the parameters listed using the UPLC gradient in Table 1.
Currently, there is a limited amount of literature reporting the type of components migrating from infant formula containers into the formula, so an investigative approach was taken.
The investigative workflow used for these series of experiments is shown in Figure 1. This type of approach can also be applied to other food-related experiments where comparisons need to be made between a control sample and a test sample. In this case, the control sample was the packaging at T = 0, and the test samples were T = 1, 2, 3, 4, 5, 6, 7, and 8 days.
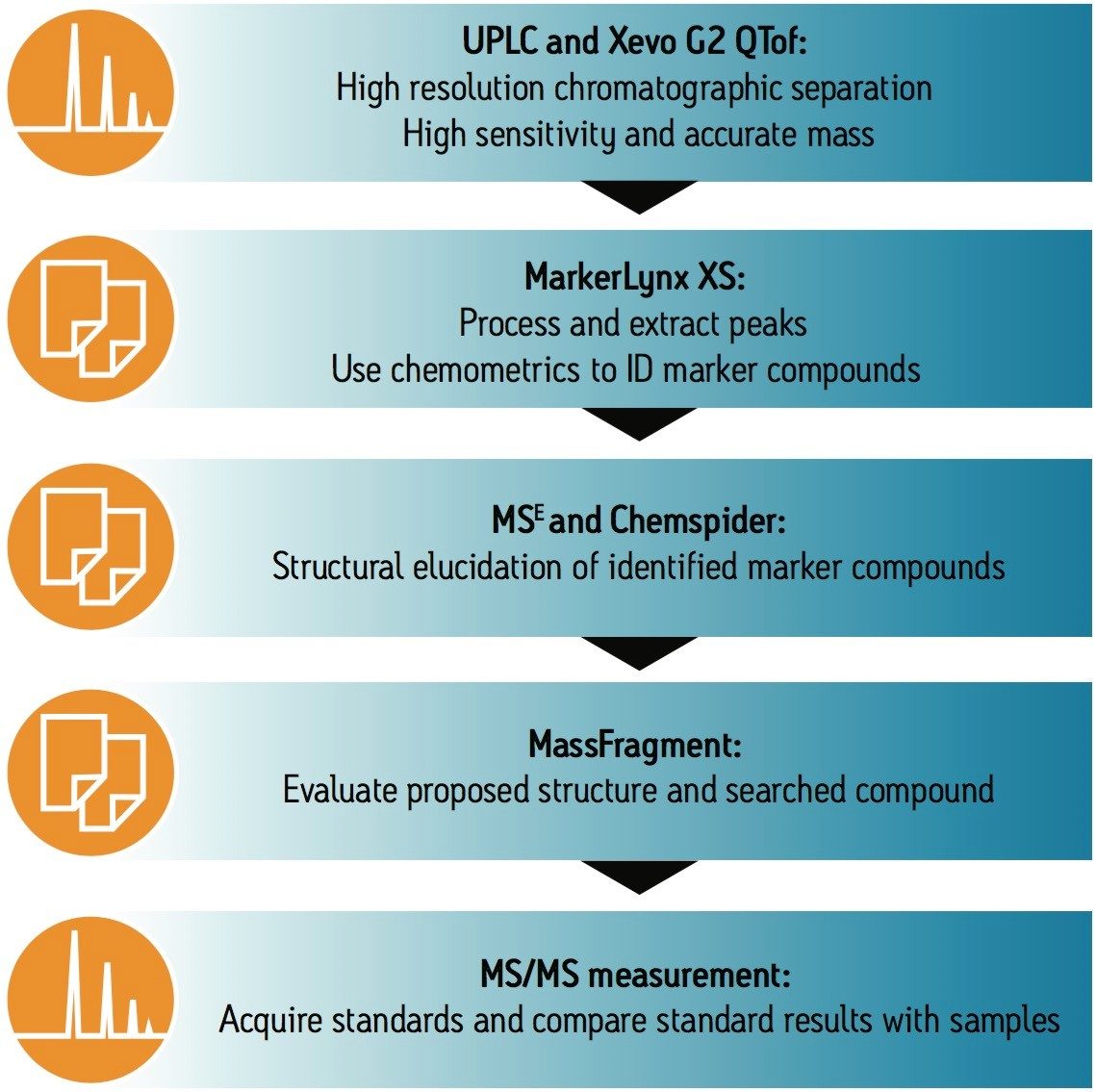
With this challenge in mind, the ACQUITY UPLC System and Xevo G2 QTof were selected for this investigation. The increased resolution of the ACQUITY UPLC System, combined with exact mass performance, MSE, and the MS/MS functionality of the Xevo G2 QTof, made this an excellent screening platform for this analysis.
After acquisition, the data were processed using MarkerLynx XS Application Manager, a chemometrics-based software package. The information was first investigated by using the Principle Component Analysis (PCA) approach to look at the differences between the packaging over the eight days of sampling. The samples can be easily compared, as shown in Figure 2.
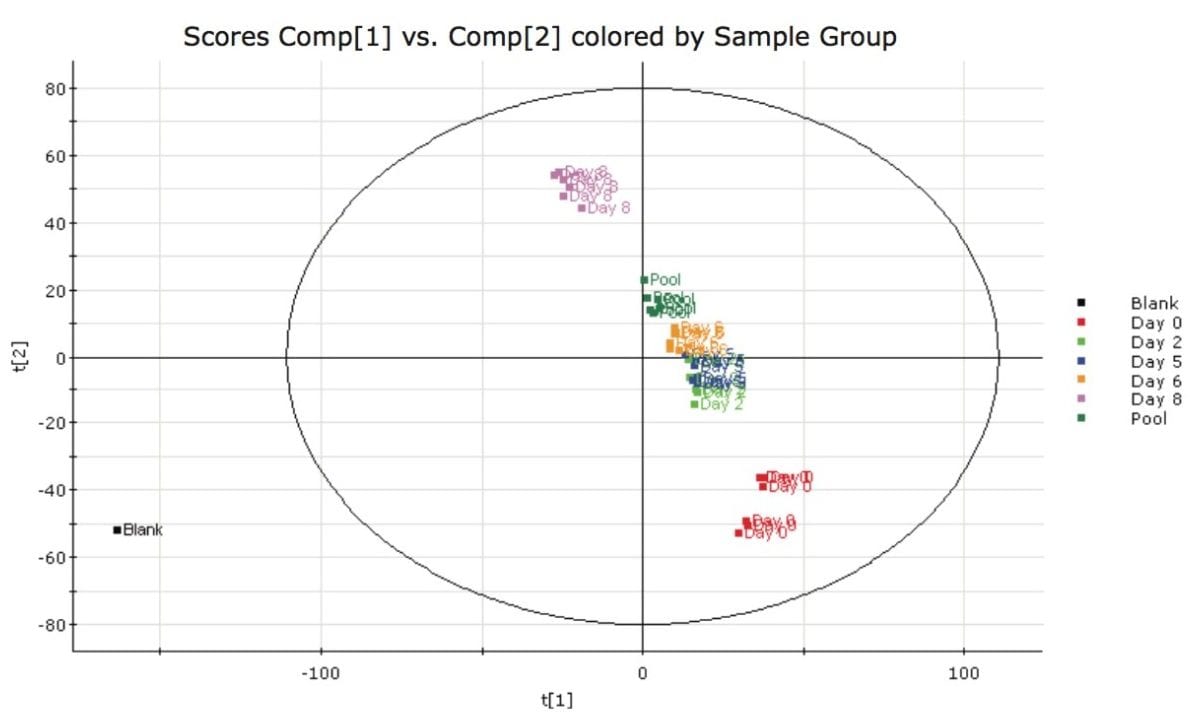
Tight groupings were observed on the days that included repeat injections (good intra-group repeatability), and large differences were observed between Day 0 and Day 8. Further investigation using the Orthogonal Partial Least Squares (OPLS) model (which is used for comparing two groups) was employed to directly compare Day 0 to Day 8. The S-Plot derived from the OPLS model illustrating the comparison between the two groups (Day 0 and Day 8) is shown in Figure 3.
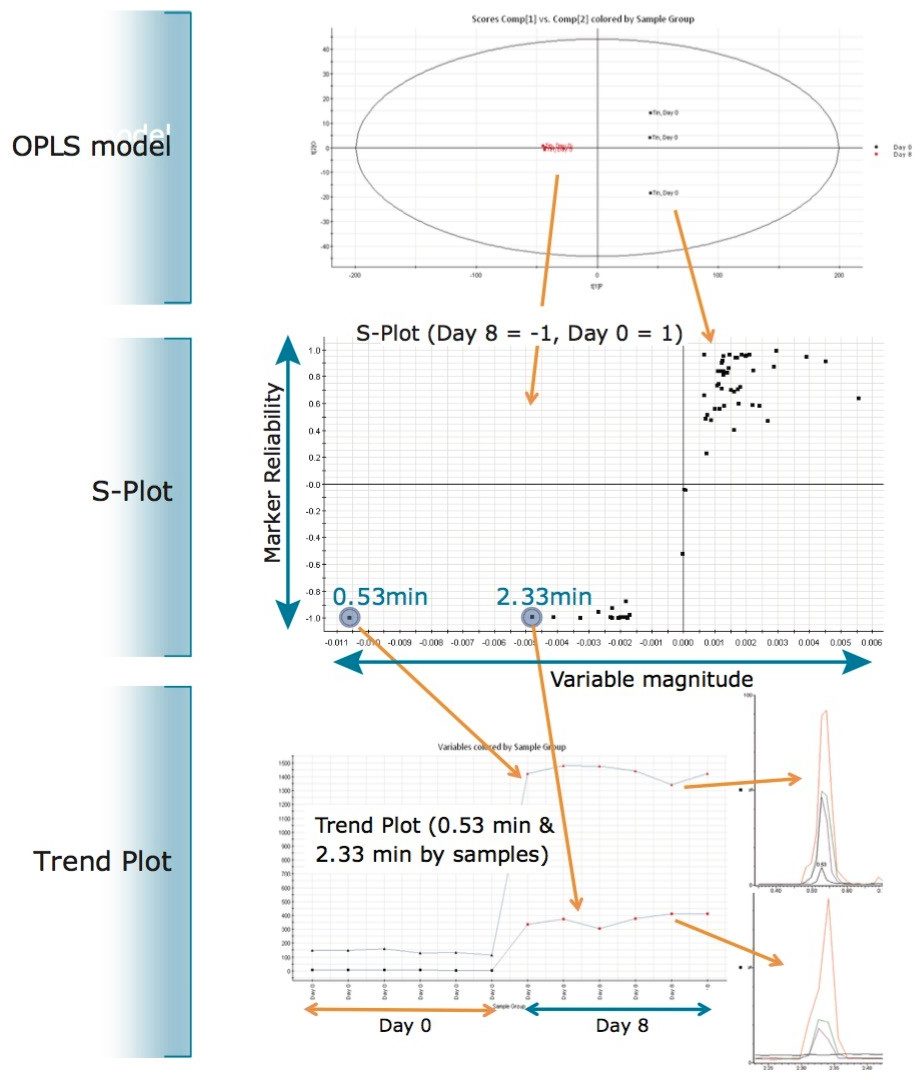
Further visualization using a trend plot shown in Figure 3 revealed that the concentrations of the two compounds, with retention times of 0.53 min and 2.33 min, increased on Day 8, compared to Day 0. The BPI chromatograms (Days 0, 2, 6, and 8) show a gradual increase in concentration of the two compounds, as shown in Figure 3. These unknowns were further investigated to elucidate the structure and identity of the compounds.
Structural elucidation is derived by utilizing the MSE data, which are routinely acquired within an acquisition run. MSE is an acquisition technique that provides a simple, unbiased, and parallel route to deliver exact mass, low energy precursor (MS) and high energy fragment ion (MSE) information from every detectable component, without the need for multiple injections.
Using the MSE data with exact mass measurement, the elemental composition of the unknown components were identified using ChemSpider (http://www.chemspider.com). The proposed structure was then evaluated using MassFragment Software, as shown in Figure 4. Combining information from MSE, ChemSpider, and MassFragment Software, the compound with a retention time of 2.33 min was identified as benzoguanamine (2,4-diamino-6-phenyl-1,3,5-triazine), with a chemical formula of C9H9N5. Benzoguanamine, which belongs to the same family as melamine, is often cross linked with saturated polyester resin, and is commonly used in can coating.
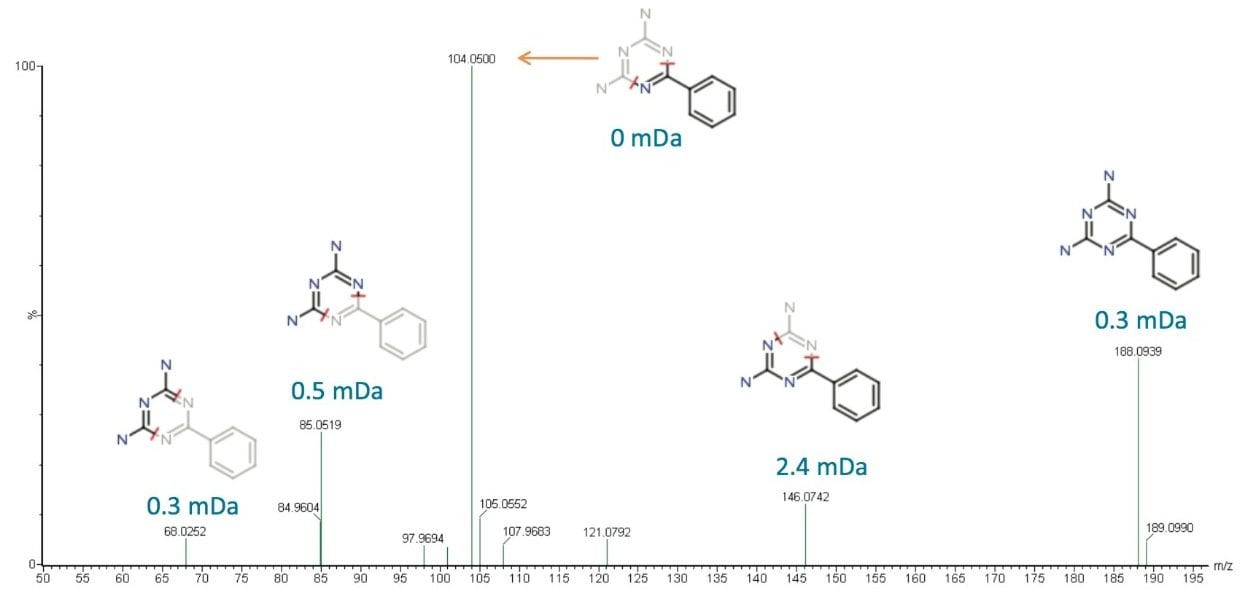
Further confirmatory analysis was performed using a commercially available benzoguanamine standard. MS/MS using Xevo G2 QTof was performed on both the standard and the Day 8 sample. The precursor mass (188.08 m/z) which corresponded to benzoguanamine produced identical fragment ion spectra in both the standard and the sample, shown in Figure 5, thus confirming the identity of the peak at 2.33 min.
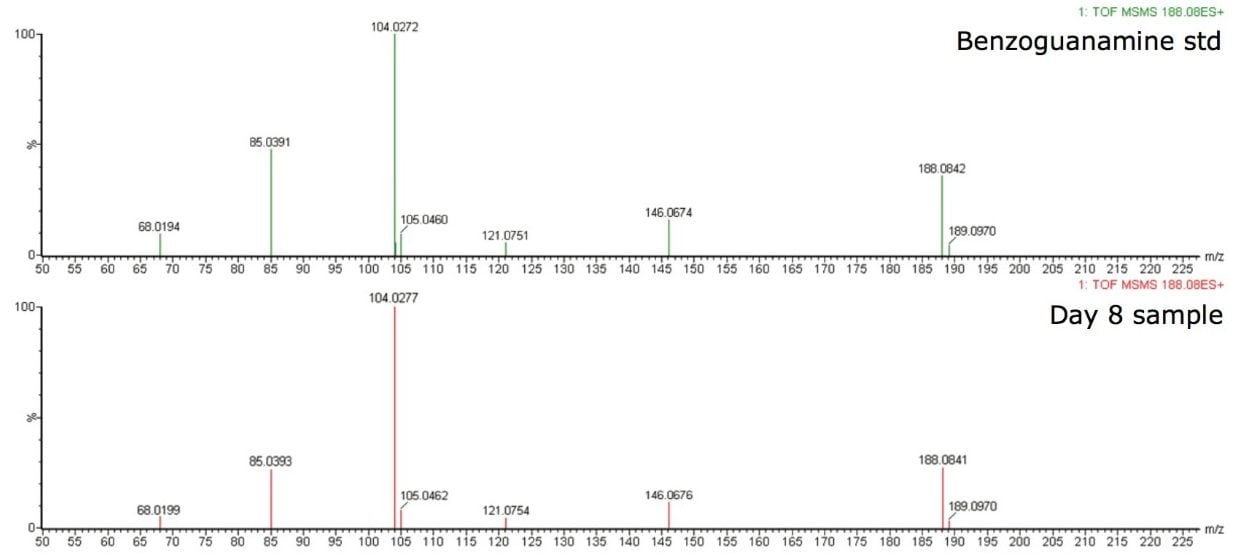
The experimental combination of ACQUITY UPLC, Xevo G2 QTof, and several data analysis software tools like MarkerLynx XS and MassFragment made possible the structural elucidation and identification of benzoguanamine from infant formula containers.
720003905, March 2011