This application note describes combining mass and UV spectral analysis allows for tracking peaks and identifying coeluting peaks in a single run.
Methods development for reversed-phase liquid chromatographic (RPLC) separations typically requires many time-consuming steps, including extensive data processing. While many screening protocols rely on UV detection, a single detection technique provides insufficient information for missed or coeluted peaks.
Isobaric compounds can be difficult to distinguish with a mass detector. In addition, minor components may be missed against background, and some important sample components may not ionize. Peak tracking with UV is not possible for compounds that lack a chromophore. Spectra may not be reliably distinguishable. It is not generally easy to recognize which spectra have been “summed” in a coelution. Due to extreme differences in concentration, the minor peak spectrum may simply disappear in a coelution. Finally, UV spectra may change with solvatochromatic effects, in particular with changes in pH.
To address some of these challenges, multiple detectors can be used for analysis of a single sample with each detection technique dependent on a different physical or chemical property of the molecule. Combining detector responses into a single software interface allows streamlined data analysis in a simplified platform.
Set of analgesics containing acetylsalicylic acid, acetaminophen, 2-acetamidophenol, acetanilide, phenacetin, and caffeine were prepared at 0.2 mg/mL in 90:10 water/acetonitrile.
|
LC system: |
ACQUITY UPLC H-Class System with ACQUITY Isocratic Sample Manager (ISM) |
|
UV detector: |
ACQUITY UPLC PDA |
|
Column: |
ACQUITY UPLC BEH C18, 1.7-μm, 2.1 x 50 mm |
|
Column temp.: |
30 °C |
|
Sample temp.: |
20 °C |
|
Solvent A: |
125mM formic acid in water |
|
Solvent B: |
125 mM ammonium hydroxide in water |
|
Solvent C: |
Acetonitrile |
|
Solvent D: |
Water |
|
Composition: |
Prepared using Auto•Blend Plus |
|
Wash solvent: |
50:50 water/ acetonitrile with 0.05% formic acid |
|
Purge solvent: |
90:10 water/methanol |
|
Seal wash: |
90:10 water/methanol |
|
Flow rate: |
0.6 mL/min |
|
Gradient: |
2-10% acetonitrile in 0.5 min, 10-50% acetonitrile in 0.3 min |
|
Wavelength: |
245 nm |
|
Sampling rate: |
20 pts/sec |
|
Time constant: |
Normal (0.1s) |
|
ISM solvent: |
90:10 water/acetonitrile 0.1% formic acid |
|
ISM split: |
10 (UPLC) |
|
ISM flow rate: |
0.5 mL/min |
|
Injection volume: |
1 μL |
|
Mass detector: |
ACQUITY QDa |
|
Ionization mode: |
ESI+, ESI- |
|
Acquisition range: |
100-250 m/z |
|
Sampling rate: |
5 pts/s |
|
Capillary voltage: |
0.8 kV |
|
Cone voltage: |
10 V |
|
Probe temp.: |
600 °C |
Empower 3 FR2
In the following study, a mixture of analgesics was analyzed on an ACQUITY UPLC H-Class System with both ACQUITY QDa and ACQUITY UPLC PDA detectors. Empower 3 Software was used as the operating software. The initial screening, at mobile phase pH of 5, resulted in the separation of five of the six components in the standard (Figure 1).
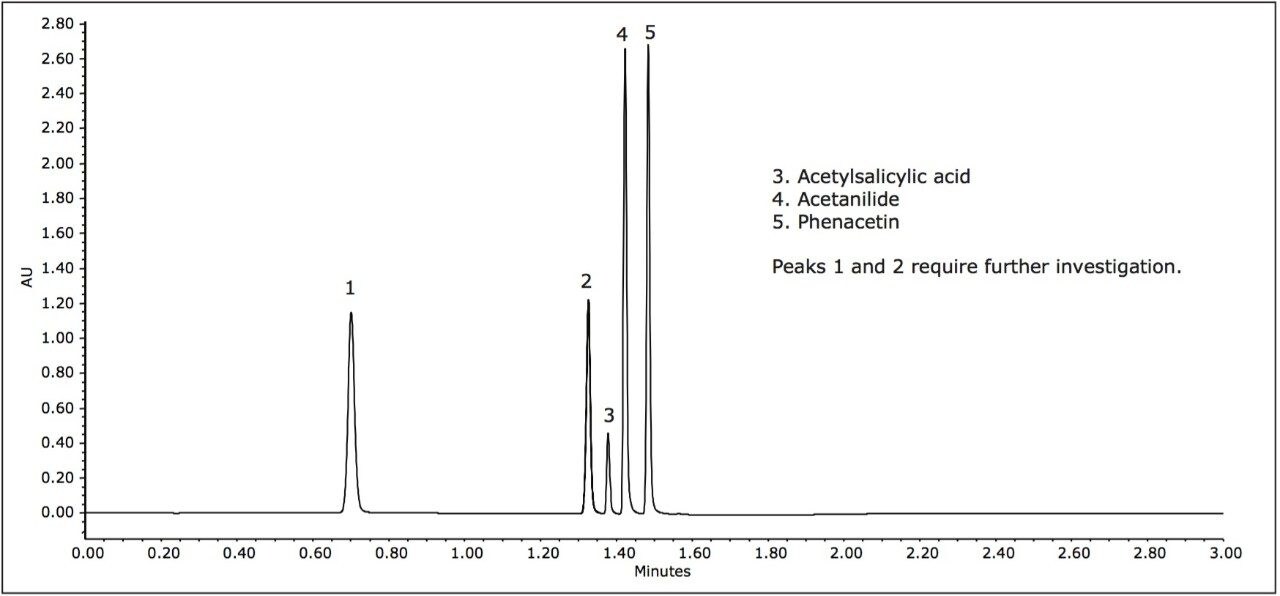
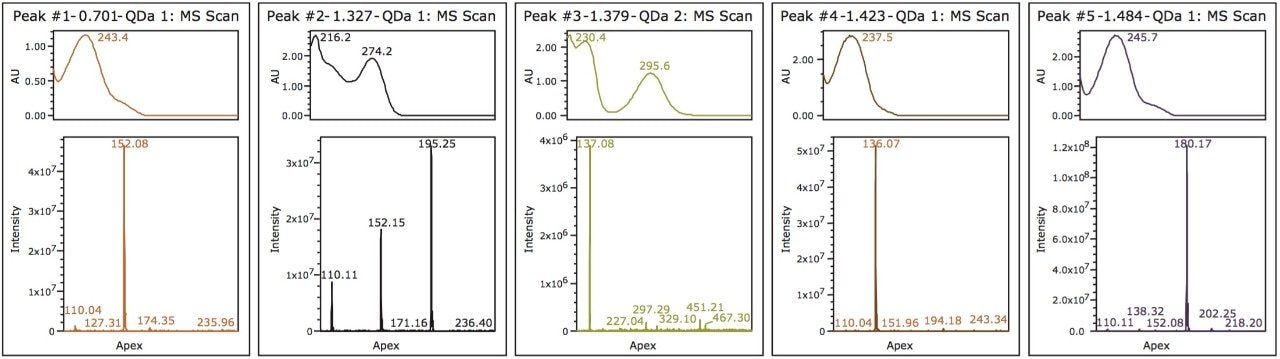
For peak tracking in the separation and to determine the presence of any coelutions, mass and UV spectral data were analyzed in tandem. The mass spectral data was used to identify those components possessing a unique mass in the mixture. For example, tentative identification of peak 4, acetanilide (m/z 136.0) and peak 5, phenacetin (m/z 180.0) could be made. Peak 3 was present in negative ionization mode and was also identified by a unique mass, acetylsalicylic acid (fragment ion at m/z 137.0). Assignment of peak 1 and 2, however, required further investigation of both mass and UV spectral data.
Two isobaric compounds (acetaminophen and 2-acetamidophenol) were present in the mixture (monoisotopic mass of 152.1). In the separation (Figure 1), both peak 1 and peak 2 contain the corresponding mass. In addition, peak 2 contained additional prominent ions, indicating potential coelutions. Therefore, while UV detection is required for peak tracking of the isobaric species, to confirm the identification, the components need to be fully resolved from all other analytes in peak 2.
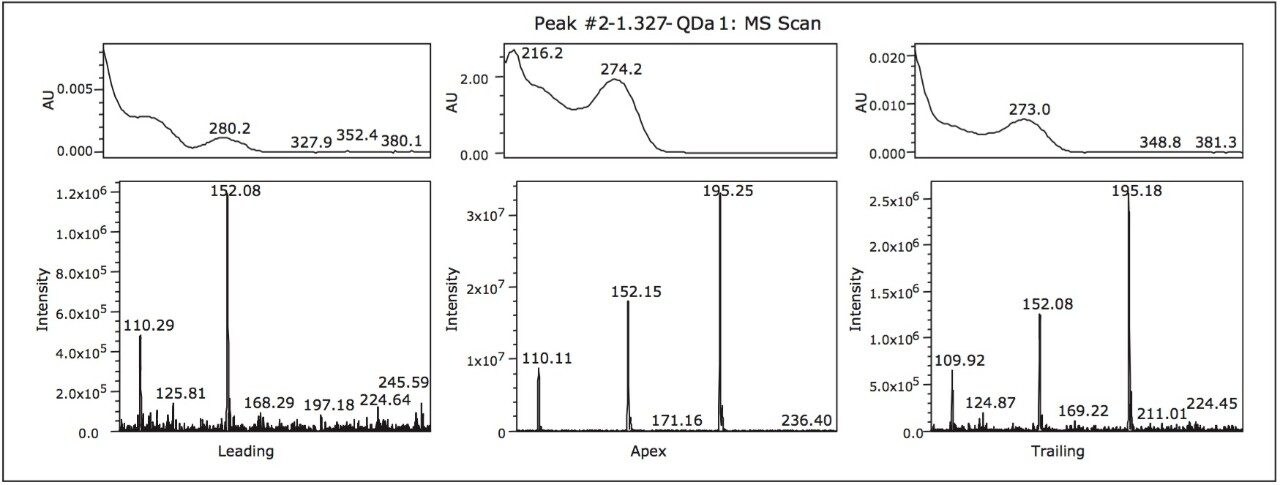
To evaluate the purity of peak 2, the mass analysis window in Empower 3 Software was used (Figure 2). Evaluation of the peak (1.3 min) reveals m/z 152 and m/z 110 to be present in the leading edge of the peak (Figure 3). In contrast, the trailing edge, while showing the presence of all three masses, reveals a different ion ratio than that of the apex. The presence of the three ions is suggestive of multiple analytes eluting together. Specifically, the different ion ratios and the absence of m/z 195 at the leading edge suggests partial chromatographic resolution. If the ratios had been constant across all time segments, the possibility of fragmentation in the source would have been likely. Given the known composition of the mixture, the predominant mass at the trailing edge of the peak can be identified as caffeine (m/z 195.0). The leading edge contains both the parent ion of the isobaric compounds (2-acetamidophenol or acetaminophen) as well as the common fragment ion m/z 110.0.1
In order to improve the resolution of the coeluting species in peak 2, the effect of mobile phase pH was evaluated. Using flexible software, the reversed-phase gradient was entered directly in pH units using Auto•Blend Plus.2 This process allowed for manipulation of pH without the need for preparing new buffer bottles.
Increased mobile phase pH altered the selectivity of the two coeluting compounds (Figure 4). Caffeine (peak B) was found to be predominant mass (m/z 195) in the later of two unresolved peaks at pH 6. At pH 7, the two species were baseline-separated with caffeine (peak B) eluting later (peak A) at 1.2 min.
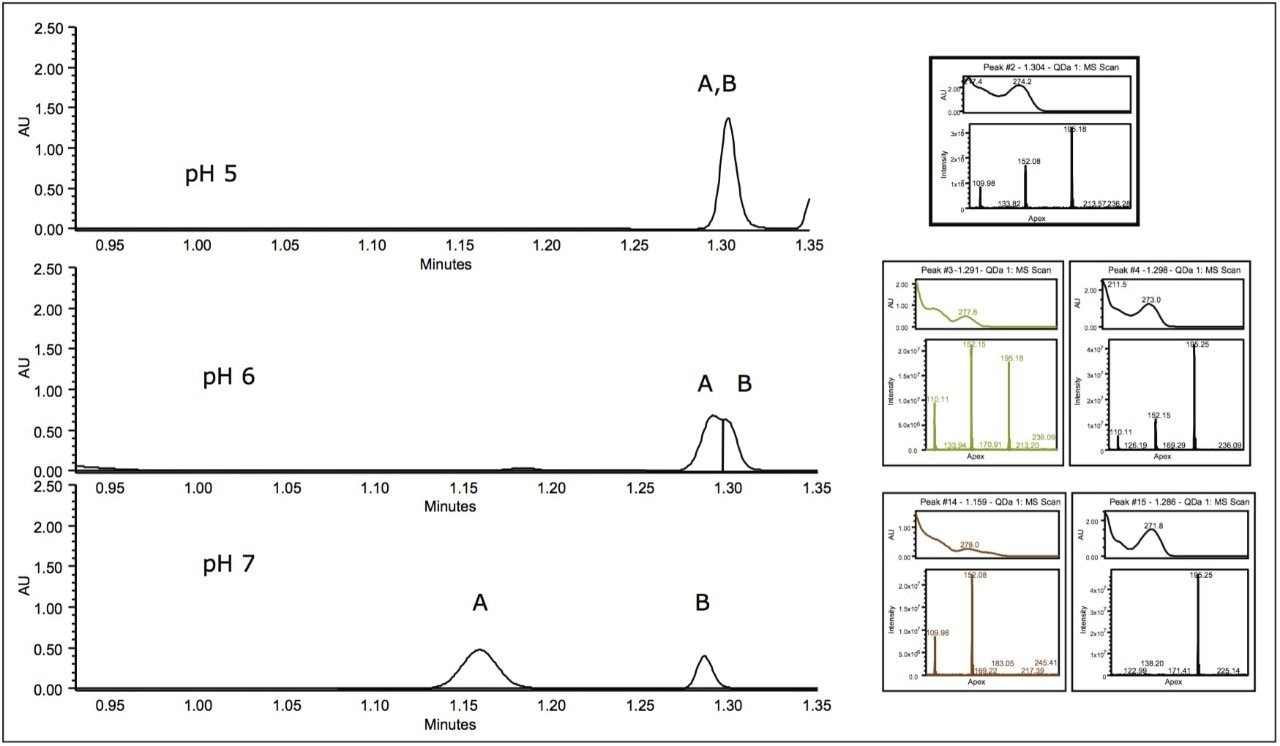
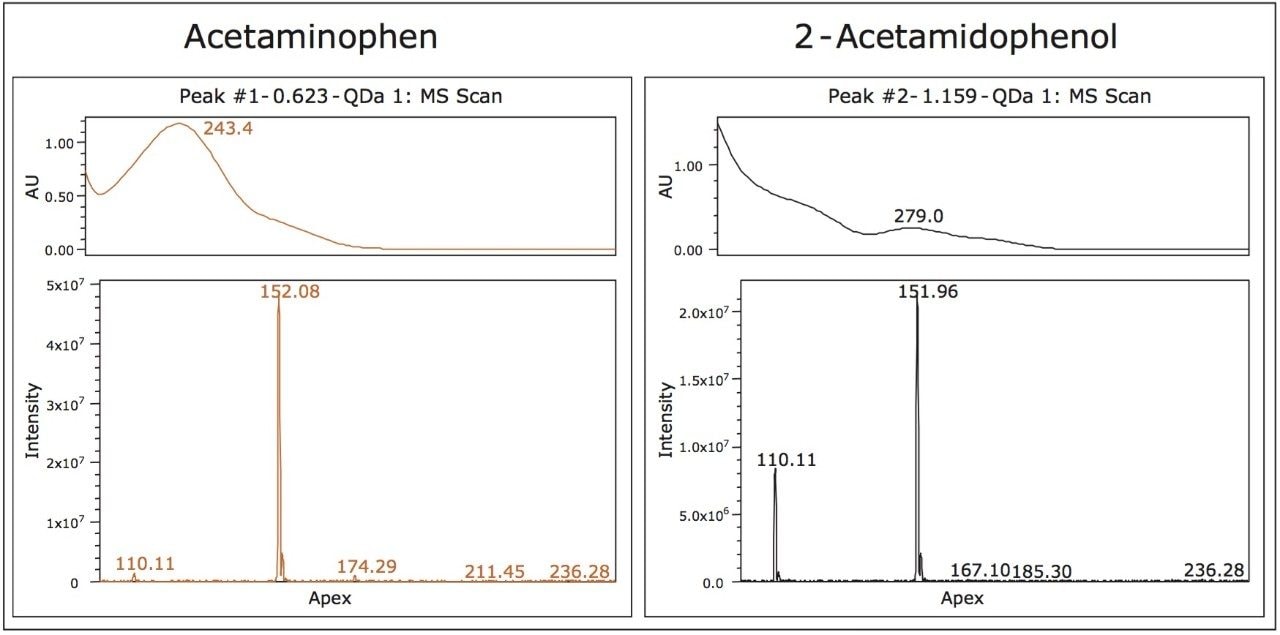
The baseline separation of one of the isobaric compounds (peak A) and caffeine (peak B) at mobile phase pH 7 (Figure 4) enables the use of the UV spectrum for peak tracking of both isobaric compounds. While the predominant mass for both peak 1 (Figure 1) and peak A (Figure 4) is the same, the peak A contains a fragment ion at 110 m/z (Figure 5). Comparison of the mass and UV spectrum for the isobaric compounds provides the information needed for peak identification: the UV spectrum of peak 1 corresponds to that of acetaminophen (λ max of 243 nm). The later eluting peak A could thus be identified as 2-acetamidophenol.
Methods development using a single detection technique can be challenging. UV spectral data can assist in peak purity and identification, however, it is difficult to perform peak tracking in the presence of coelutions. Mass spectral data can be used to match chromatographic peaks to items on a short list of compounds, but the presence of isobaric compounds can require additional information: complete separation is typically required and not only allows for immediate identification based on the UV but also more reliable quantification.
Typically, identifying these phenomena requires further analyses. For simplified methods development, a chromatographic system using both UV and mass detection and a streamlined software platform can be combined to evaluate peak purity and assist in the identification of coelutions in a single run.
720004846, November 2013