
This application note presents the versatility of direct injection using the ACQUITY I-Class UPLC System with the Xevo TQ-S Mass Spectrometer for the analysis of diquat and paraquat in tap water and bottled water. The limit of detection in this study was 50 ppt, which is below the European Union Directive LOD of 100 ppt. The high sensitivity of Xevo TQ-S enabled excellent quantitation using a 100-μL injection without sample extraction or concentration prior injection. The recovery data showed good results with excellent RSD’s below 8% for both water samples.
Crop protection in countries around the globe is usually associated with the use of a wide range of pesticides, insecticides, or herbicides. These agricultural products can potentially have harmful effects on the environment and impact the health of both humans and animals. Despite the risk, they are a crucial part of the global economy1 For example, the use of herbicides is important to control the growth of weeds, for if not suppressed weeds can reduce crop yields up to 80%.2 In the herbicides family the bipyridyls are used extensively in agriculture to control broadleaf and aquatic weeds. The most common bipyridyls are diquat and paraquat. They constituted the largest share of the global market until recently overtaken by glyphosate.3 Due to their high efficiency as pre-harvest desiccants and defoliants, diquat and paraquat are also classified as highly toxic.4 The World Health Organization (WHO) has classified these compounds as moderately hazardous.5 Even with a half-life in water of 48 hours, accidental or intentional ingestion can have serious health effects. For drinking water, the U.S. Environmental Protection Agency (U.S. EPA) has established a maximum contaminant level of 20 ppb for diquat and a desired goal of 3 ppb for paraquat6 (not EPA regulated). The European Union (EU) has not regulated the levels of these compounds specifically in drinking water and continues to apply the value of 0.1 ppb.7
The analysis of bipyridylium herbicides can be difficult mainly because they are cationic molecules. Their inherent high polarity and positive charge, require the use of ion pairing additives when analyzing quaternary amines by reversed-phase chromatography. The U.S. EPA method 549.2 utilizes reversed-phase chromatography with ion pairing for the separation of diquat and paraquat using UV detection.8 Ion pairing agents are typically avoided with ESI-MS applications owing to suppression of the ionization in the MS source. For MS applications, HILIC has provided suitable chromatography without the requirement of ion pairing agents.9 However, recent advances in MS sensitivity have made the direct analysis of trace-level contaminants in water attainable and very attractive. The possibility of removing laborious and time-consuming solid phase extraction and sample concentration is highly desirable. Direct injection of an aqueous sample for RP chromatography is ideal as the sample matrix is similar to the initial mobile phase conditions. For HILIC, a water sample would first require dilution with the organic solvent.
This application note presents the analysis of diquat and paraquat herbicides in drinking water by direct injection using a volatile ion pairing reagent (heptafluorobutyric acid-HFBA), RP-UPLC, and the highly sensitive Xevo TQ-S.
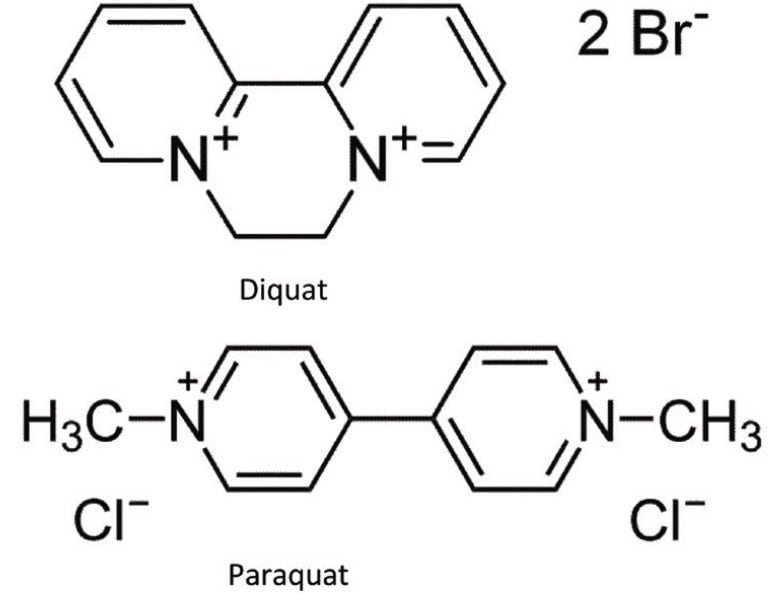
Diquat and paraquat standards were purchased from Sigma Alrich (St-Louis, MO, USA). HFBA (HPLC grade) was purchased from Thermo Scientific (Rockford, IL). MilliQ water was used to produce calibration standards. The water samples were collected from bottled and in-house tap water. The chemical structure and MRM conditions used for the quaternary herbicides are listed in Figure 1 and Table 1, respectively. MRM transitions stored in the Quanpedia database were selected for analysis. Chromatographic separation was performed on Waters ACQUITY UPLC System equipped with an ACQUITY UPLC BEH C18 2.1 x 30 mm Column. A one -minute linear water/methanol gradient with 10 mM HFBA was used. The detection was performed using a Xevo TQ-S.
|
UPLC system: |
ACQUITY UPLC |
|
Runtime: |
3.0 min |
|
Column: |
ACQUITY UPLC BEH C18 2.1 x 30 mm, 1.7 μm |
|
Column temp.: |
25 °C |
|
Mobile phase A: |
10 mM HFBA in water |
|
Mobile phase B: |
10 mM HFBA in methanol |
|
Elution: |
1 minute linear gradient from 2% (B) to 95% (B) |
|
Flow rate: |
0.6 mL/min |
|
Injection volume: |
100 μL |
|
MS system: |
Xevo TQ-S |
|
Ionization mode: |
ESI positive |
|
Capillary voltage: |
3.0 kV |
|
Cone voltage: |
50.0 V |
|
Source temp.: |
140 °C |
|
Desolvation temp.: |
550 °C |
|
Desolvation gas: |
1100 L/hr |
|
Cone gas: |
50 L/hr |
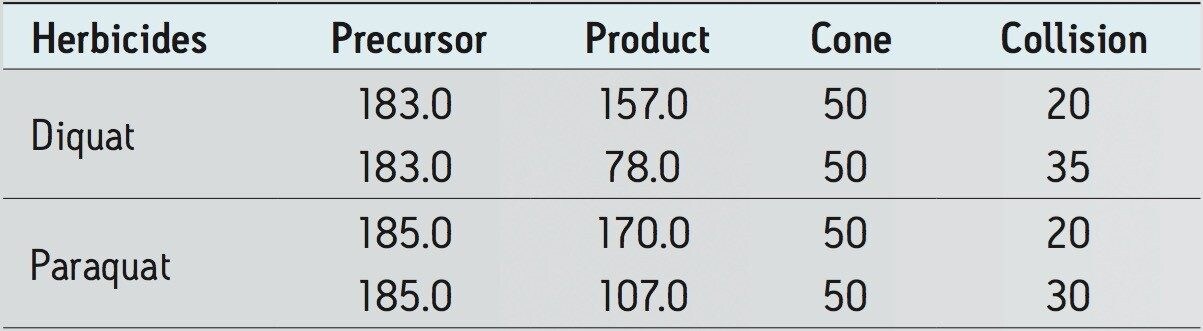
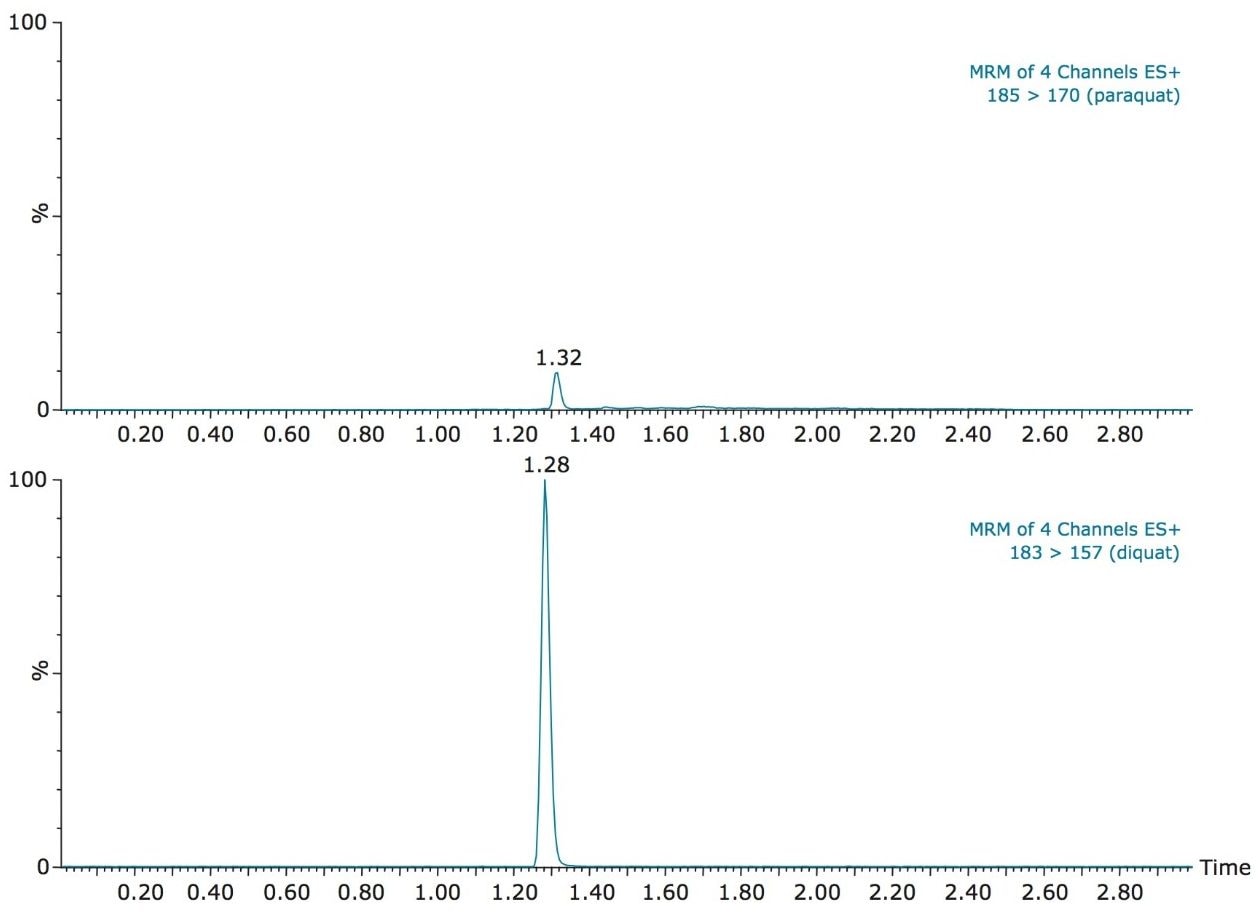
With the StepWave ion optics, Waters Xevo TQ-S offers unsurpassed performance for trace-level analysis. The high sensitivity allows for the option to bypass the tedious sample concentration requirement associated with trace-level detection of contaminants in drinking water. With this high level of sensitivity, a clean water sample can be pre-concentrated directly on column by using a direct injection technique with the ACQUITY UPLC System. As shown in Figure 2, diquat and paraquat gave well-defined Gaussian peak shapes on the RP column. The vertical axes are linked in Figure 2 and show the difference in response of the two analytes. Even with the lower response seen for paraquat compared to diquat, the required levels of quantification for both compounds were achieved.
Using the direct injection protocol, the quantification of bottled and tap water was measured against a calibration curve generated using standards made in MilliQ water. In this case, external calibration showed excellent results and an internal standard was not deemed necessary. As shown in Figure 3, the calibration curves for diquat and paraquat for tap water showed excellent linearity from 50 ppt to 100 ppb, with r2 of 0.997 and 0.995 for diquat and paraquat, respectively. The recoveries for a 1 ppb spike are shown in Table 2, with recoveries in the range of 75% to 107%. The relative standard variation (RSD’s) for diquat and paraquat was below 8% in both water samples.
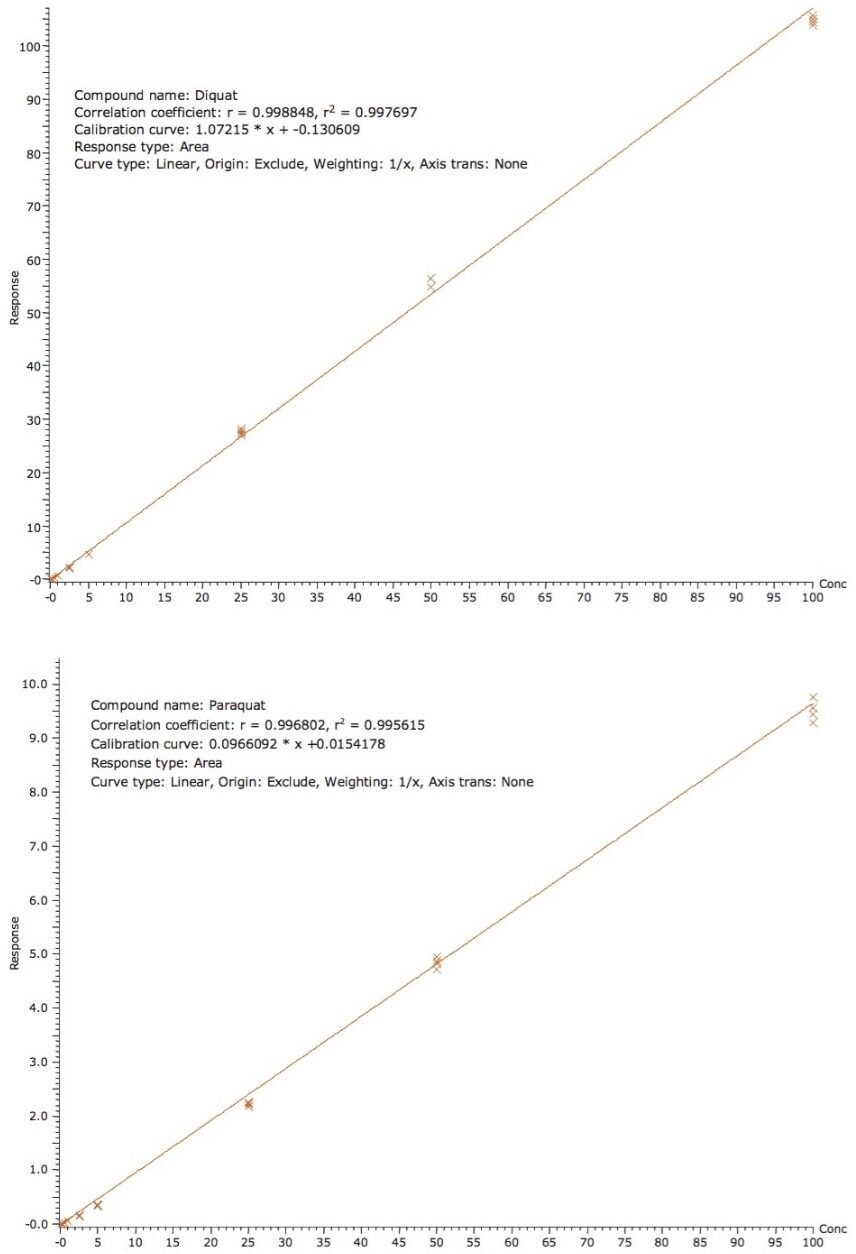
Figure 3A. Calibration curve for diquat from 50 ppt to 100 ppb.
Figure 3B. Calibration curve for paraquat from 50 ppt to 100 ppb.
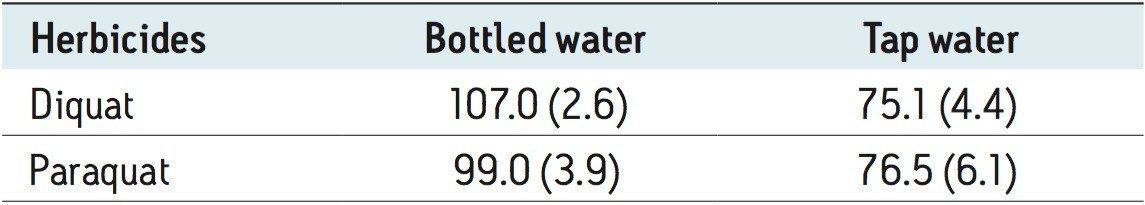
In this application, since the ion pairing agent was added to both the mobile phases (aqueous and organic) and the sample, the purity of HFBA was crucial. During the development phase, the 185 → 170 m/z MRM transition for paraquat showed an interferent near the expected retention time of paraquat. It also showed high background levels which made it difficult to quantify paraquat below 500 ppt. This issue was attributed to the ion pair additive, most likely due to a lower purity grade that was employed. With a higher purity grade, the interferent was eliminated and the background noise was reduced to a satisfactory level. As a consequence, the limit of detection (LOD) of 50 ppt was achieved and the MRM chromatograms are presented in Figure 4 for bottled water. The ion ratios for both diquat and paraquat, calculated from the quantification and the confirmation MRM transitions (Figure 5) showed good correlation between the standard and spiked samples, further supporting the applicability of the direct injection method.

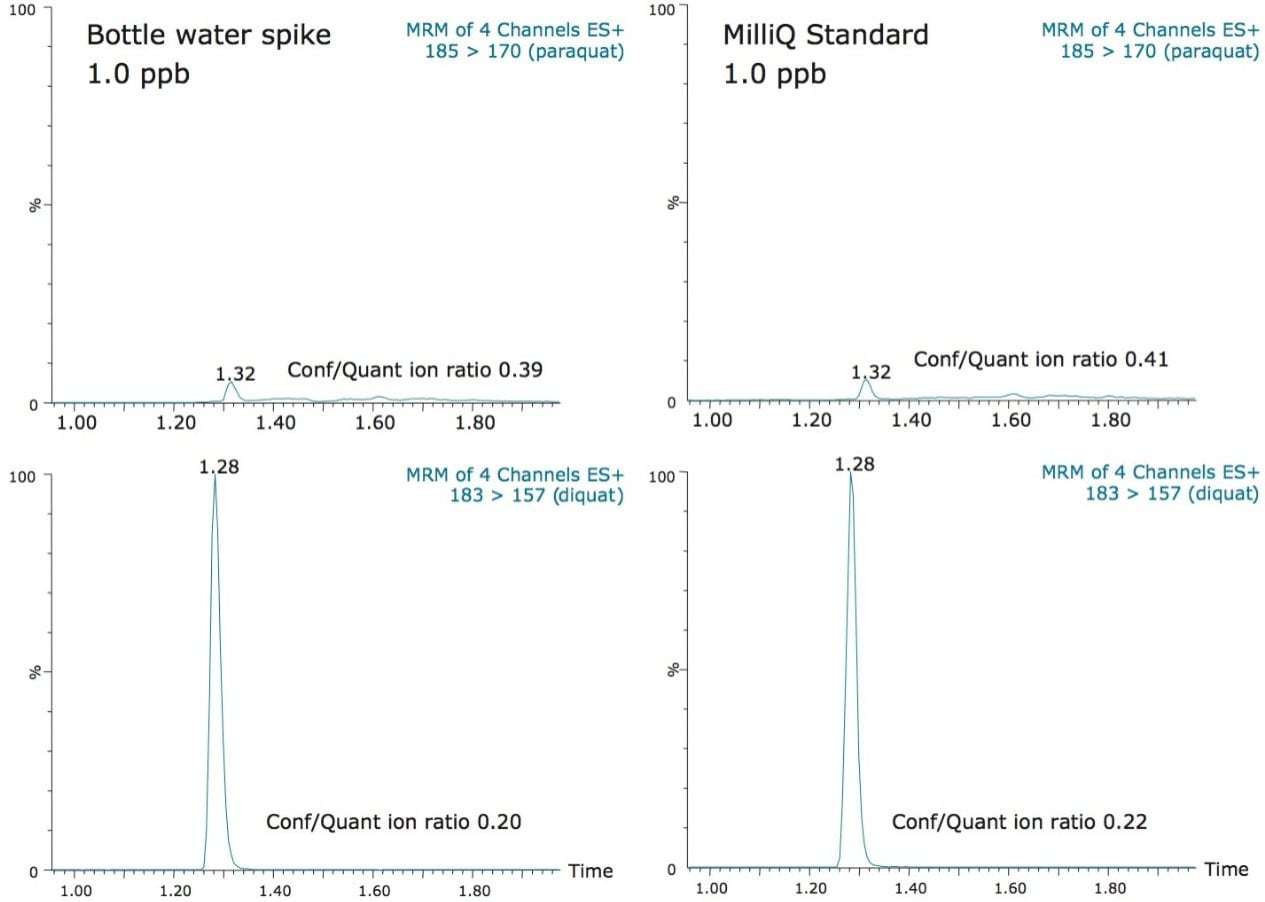
The direct injection approach is very efficient in term of speed and ease of use. However, the technique is not immune to potential situations which could affect the analytical performance over extended periods of time. The repeated injection and high injection volume of unfiltered and un-extracted samples could lead to peak distortion. During lifetime and robustness studies, the peak shape and column backpressure are excellent indicators of the column’s overall performance. In this application, as seen in Figure 6, the peak shape of diquat and paraquat showed no noticeable distortion between the first and 250th injection. The initial column backpressure readout before injection of the first sample was recorded at 3500 psi. After 250 injections of tap water samples, the initial column backpressure shows a reading of 3900 psi, an increase of 400 psi. The key feature for quantification remains for the target analyte to elute with a Gaussian peak shape throughout the analysis.
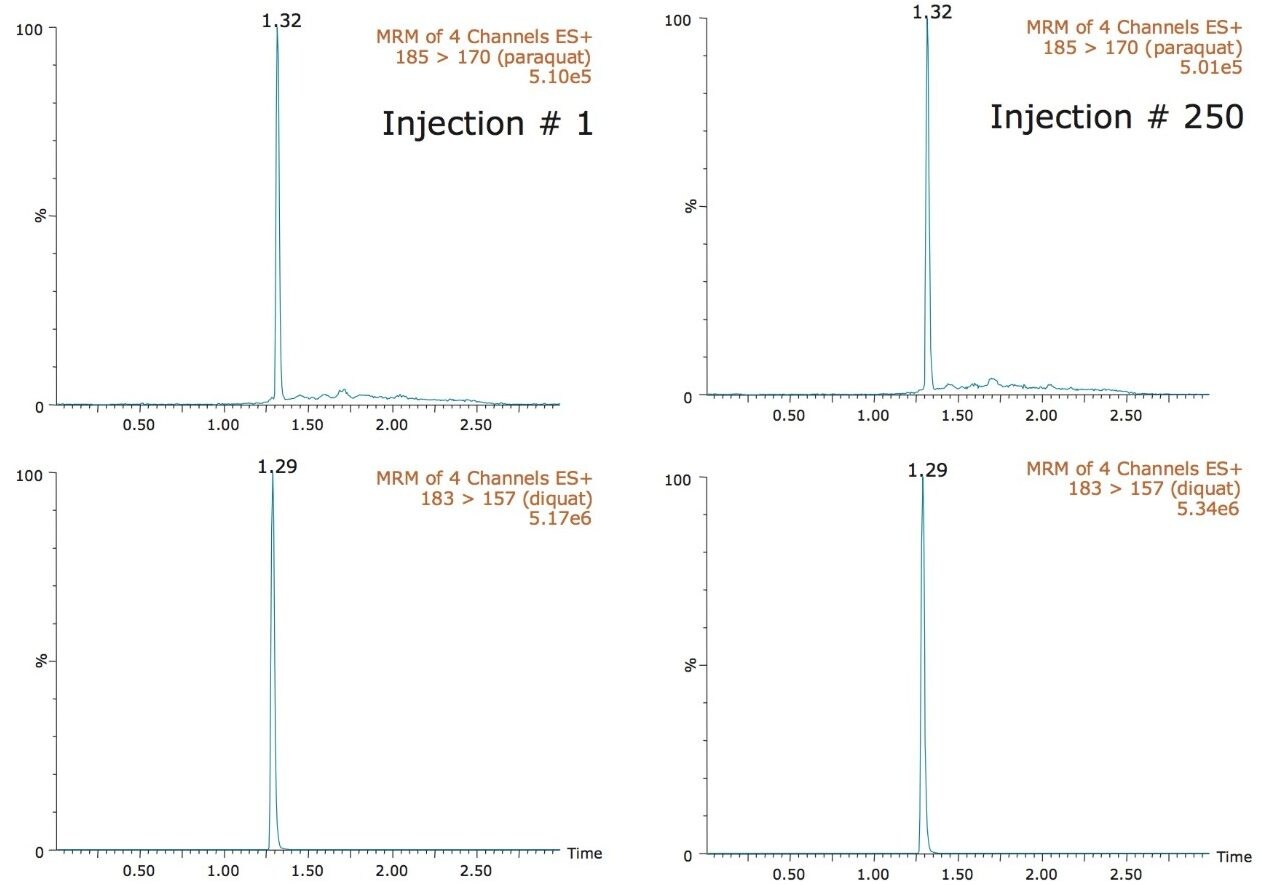
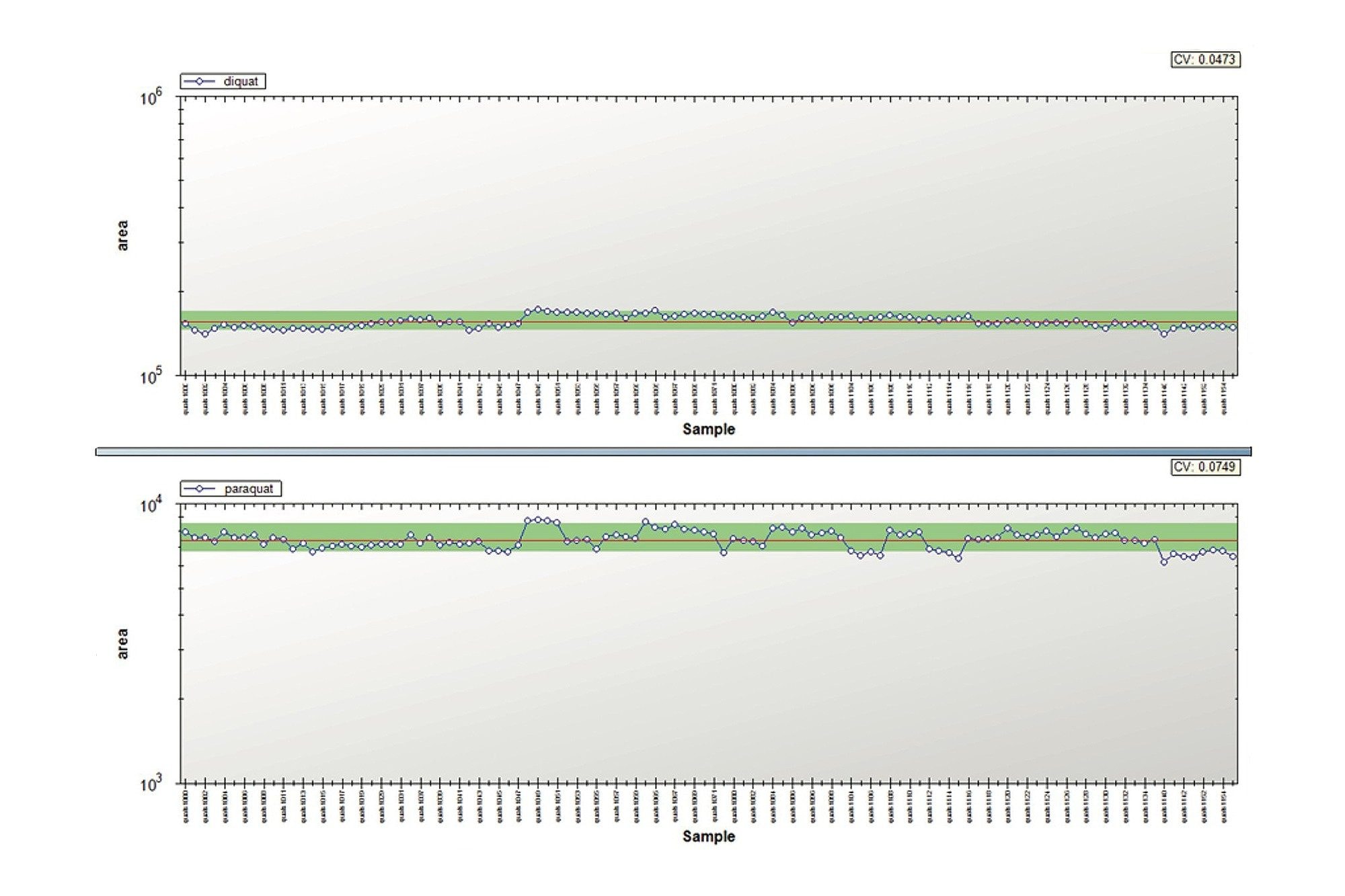
In this application, the lifetime chromatograms for diquat and paraquat showed no signs of peak distortion and the RSDs on the quantification results were below 5%. Therefore, the small backpressure increase recorded for the tap water samples did not influence the overall analytical performance during this study. The TrendPlot profile report for diquat and paraquat are shown in Figure 7. As it can be seen in Figure 7, the TrendPlot shows excellent linearity for both compounds with RSDs at 4.7% and 7.5% for 100 injections, respectively.
This application note has demonstrated the versatility of direct injection using the ACQUITY I-Class UPLC System with the Xevo TQ-S Mass Spectrometer for the analysis of diquat and paraquat in tap water and bottled water. The limit of detection in this study was 50 ppt, which is below the European Union Directive LOD of 100 ppt. The high sensitivity of Xevo TQ-S enabled excellent quantitation using a 100-µL injection without sample extraction or concentration prior injection. The recovery data showed good results with excellent RSD’s below 8% for both water samples.
720004770, August 2013