
This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates the use of Empower 3 ICH Impurity Processing for streamlining the analysis of impurities of Ziprasidone HCl by defining allowable threshold limits and quickly identifying results above the limits.
Utilizing the Empower 3 ICH Impurity Processing enables users to define allowable threshold limits for impurities and quickly identify whether results pass these limits.
Impurity profiling including identity and quantity in the drug substances or drug products are a requirement with which every manufacturer must comply. Impurities that develop from the active pharmaceutical ingredient (API) during the formulation and development process of drug product need to be assessed quickly and accurately. Presence of the impurities can compromise safety and efficacy of the end pharmaceutical product and must be effectively monitored. The International Committee for Harmonization (ICH) has published guidelines on impurities in drug substances1 and drug products,2 providing allowable threshold limits for impurities to monitor safety.
The Empower 3 ICH Impurity Processing function simplifies quantitative analysis by quickly identifying impurities above the ICH allowable limits defined by the user. Empower Software automatically compares the calculated amount of impurities against the limits and flags any failing results. This allows quick evaluation of the pharmaceutical products for safety during formulation or release testing.
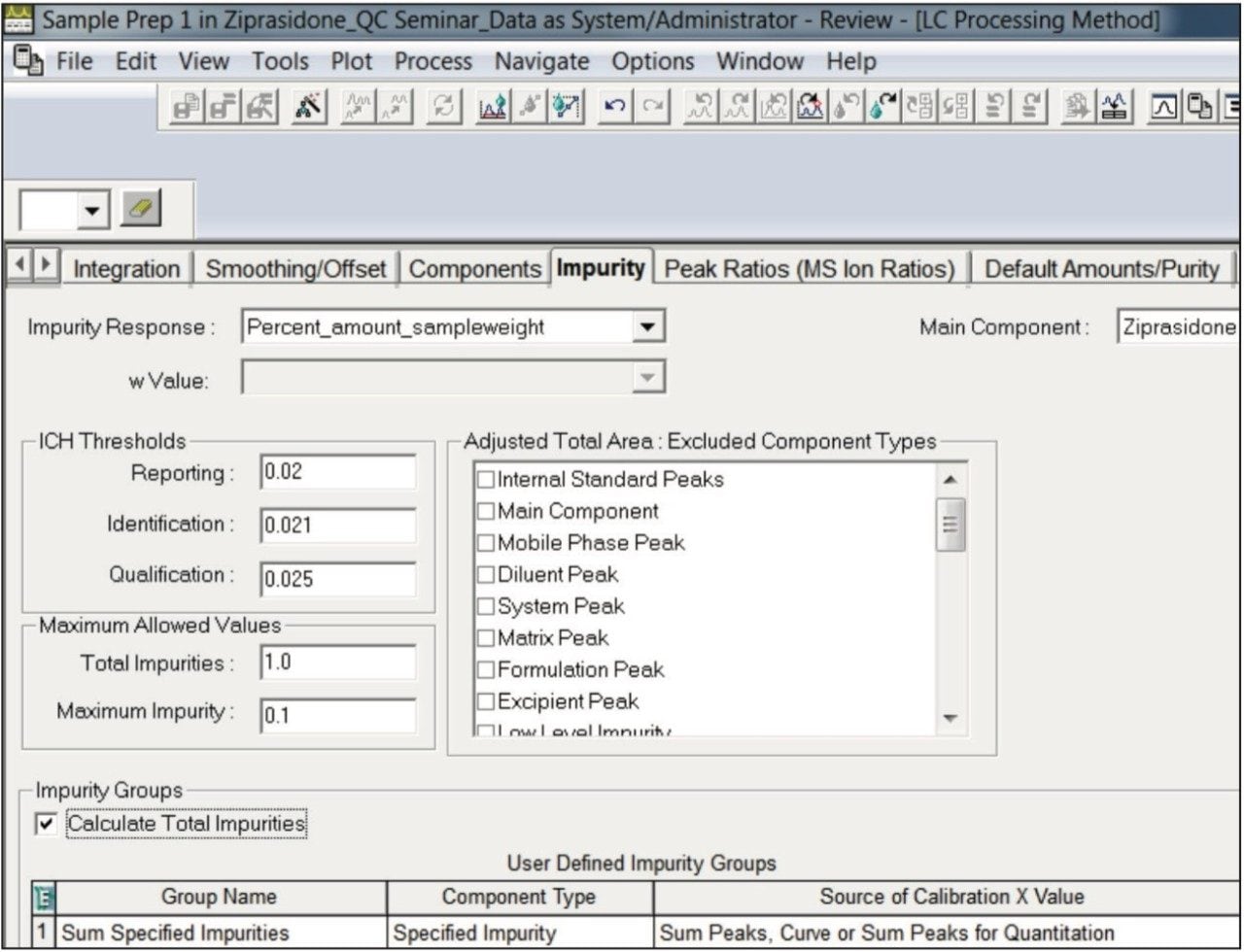
This technology brief illustrates use of Empower 3 ICH Impurity Processing for streamlining quantitative analysis of impurities of Ziprasidone HCl in a capsule formulation.
The UPLC method for Ziprasidone HCl and its USP-specified related substances was previously developed in the Waters application note 720004639EN.3 Empower 3 ICH Impurity Processing was used to streamline the quantitative analysis of related substances in the Ziprasidone HCl capsule formulation by defining the ICH threshold limits and identifying results that did not pass these limits. First, we specified the formula for calculating quantity of impurities or impurity response in the Empower processing method (Figure 1). Next, we defined the ICH thresholds for reporting, identification, and qualification of impurities. Then, we specified maximum allowed values for the total impurities and maximum impurity. In this example, we selected more stringent criteria compared to the ICH guidelines to demonstrate the workflow of identifying and flagging peaks that exceed impurity threshold limits. We used a reporting threshold of 0.02% of API. This is lower than the ICH guidelines for reporting impurities in new drug products of 0.1% based on maximum daily dosage of ≤1 g.2 In addition, we can select tighter limits than the generic ICH thresholds for each specified impurity of the pharmaceutical product. Furthermore, we can group specific types of impurities to calculate their total.
Finally, we processed the chromatographic data to determine quantity of impurities. The data (Figure 2A) shows that two impurities, compounds A and B, were detected in the capsule sample, using Empower’s proprietary peak detection algorithm ApexTrack. Empower Software quickly identified the out-of-specification (OOS) result for compound A (flagged in red), as this value was above the threshold limits defined in the processing method (Figure 2B). The results for analysis of impurities can be easily reported (Figure 3). The report template is customizable to tailor the reporting requirements for each user’s needs and can facilitate the use of electronic signatures as shown in Figure 3.
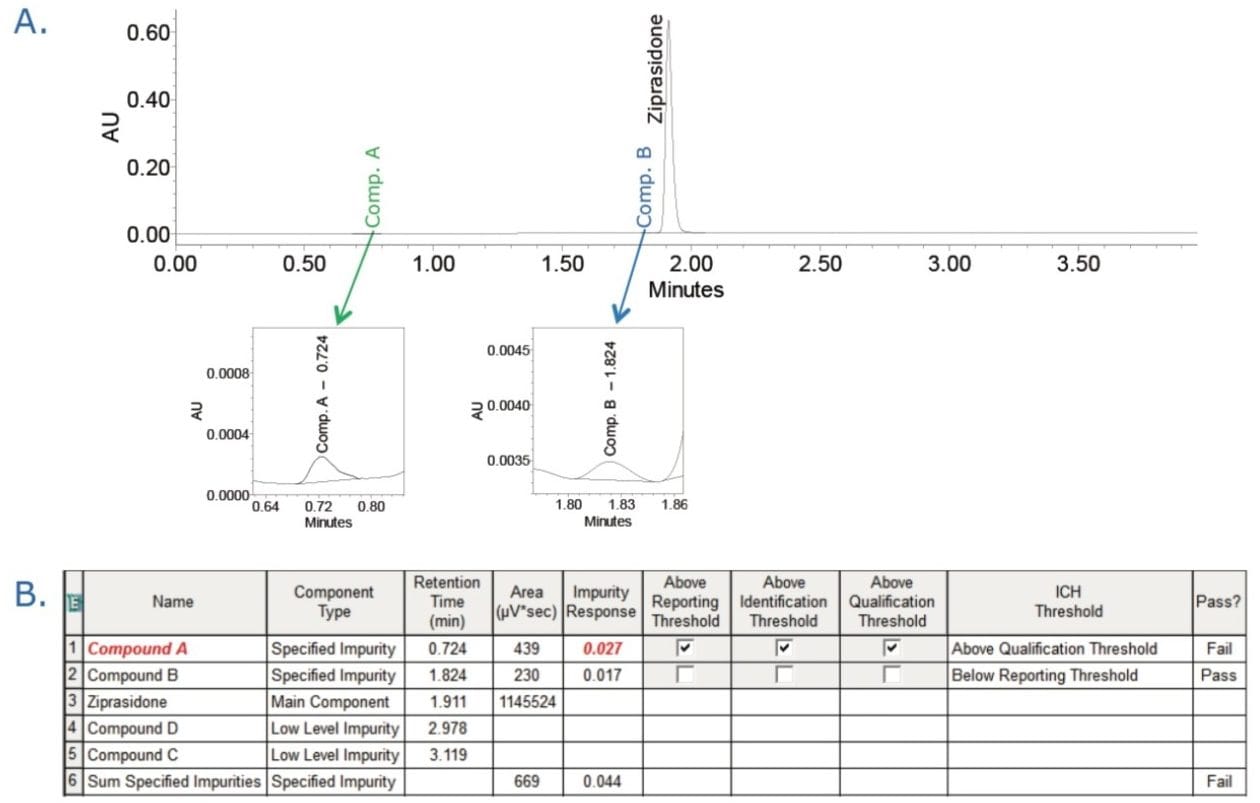
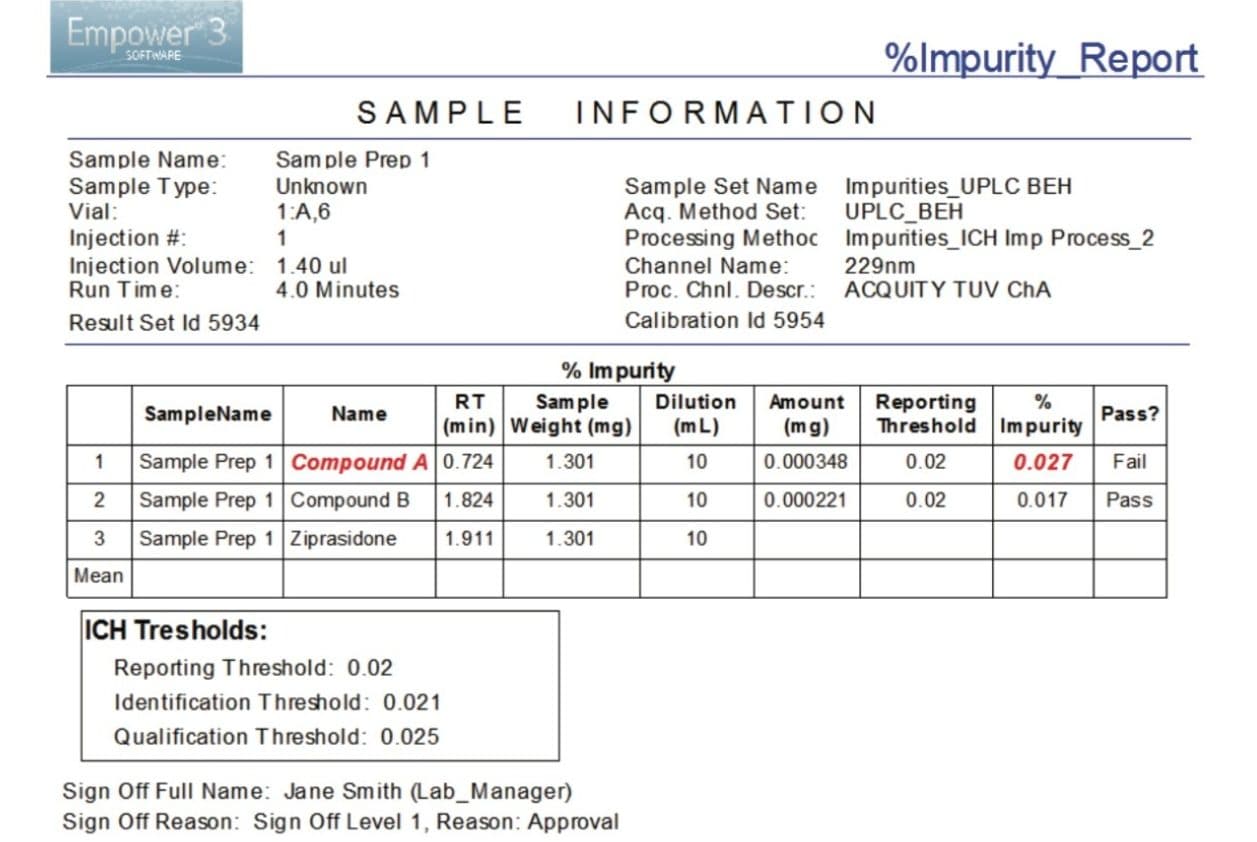
Utilizing the Empower 3 ICH Impurity Processing enables users to define allowable threshold limits for impurities and quickly identify whether results are within these limits. The threshold limits defined by the user are clearly displayed by Empower during data review or in a report. This reduces the time and potential errors associated with manual verification and enhances the confidence that any results above the threshold limits are quickly identified.
Overall, Empower 3 ICH Impurity Processing is a powerful (yet simple) tool that can be utilized by any laboratory to monitor levels of impurities during formulation or release testing of the pharmaceutical products and streamline their quantitative analysis protocols.
720005180, September 2014