
This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates the capabilities of Empower 3 Software for scoring chromatographic separations using custom calculations and custom reports.
Empower 3 Chromatography Data Software streamlines method development with custom calculations and custom reporting.
During the development of chromatographic methods, it is important to set separation goals and evaluate data in an unbiased manner. Typically as different variables are tested in method development, the scientist may rely on visual inspection of chromatograms or criteria to select the best conditions to move forward. In addition to being time consuming, this technique imparts skill level and expertise bias to the evaluation, easily resulting in the optimal result being overlooked. By contrast, a welldefined chromatogram scoring method allows all users to choose the best conditions for their method regardless of their level of experience, relying on metrics instead of judgement alone.
Empower 3 Software is a flexible chromatographic data system that allows users to perform many calculations within the data system itself, minimizing user errors in transcription and enabling the laboratory and its users to maintain compliance. Coupling custom calculations with customized reporting allows users to calculate and view only those parameters that are important to them, and to select the appropriate method conditions to move forward.
This technology brief illustrates the use the ACQUITY UPLC H-Class System with PDA and ACQUITY QDa detectors for method development using a systematic protocol. Results from each phase of the development process were scored using Empower Custom Calculations and Custom Reports for easy selection of optimal conditions for further study.
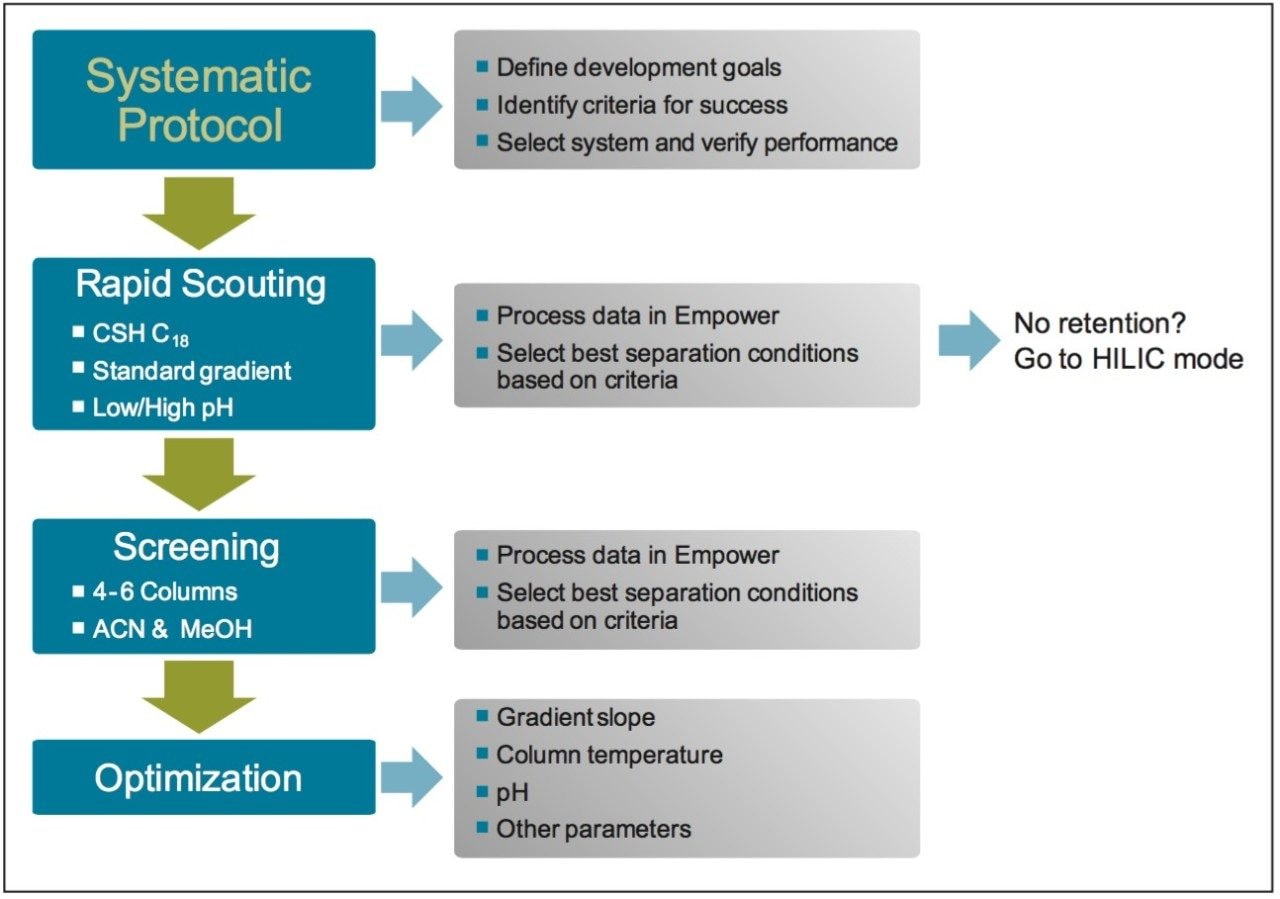
A systematic screening protocol was used for developing chromatographic methods (Figure 1). The protocol is designed to address factors of retentivity and selectivity by adjusting parameters to achieve optimal resolution of the components in a mixture.
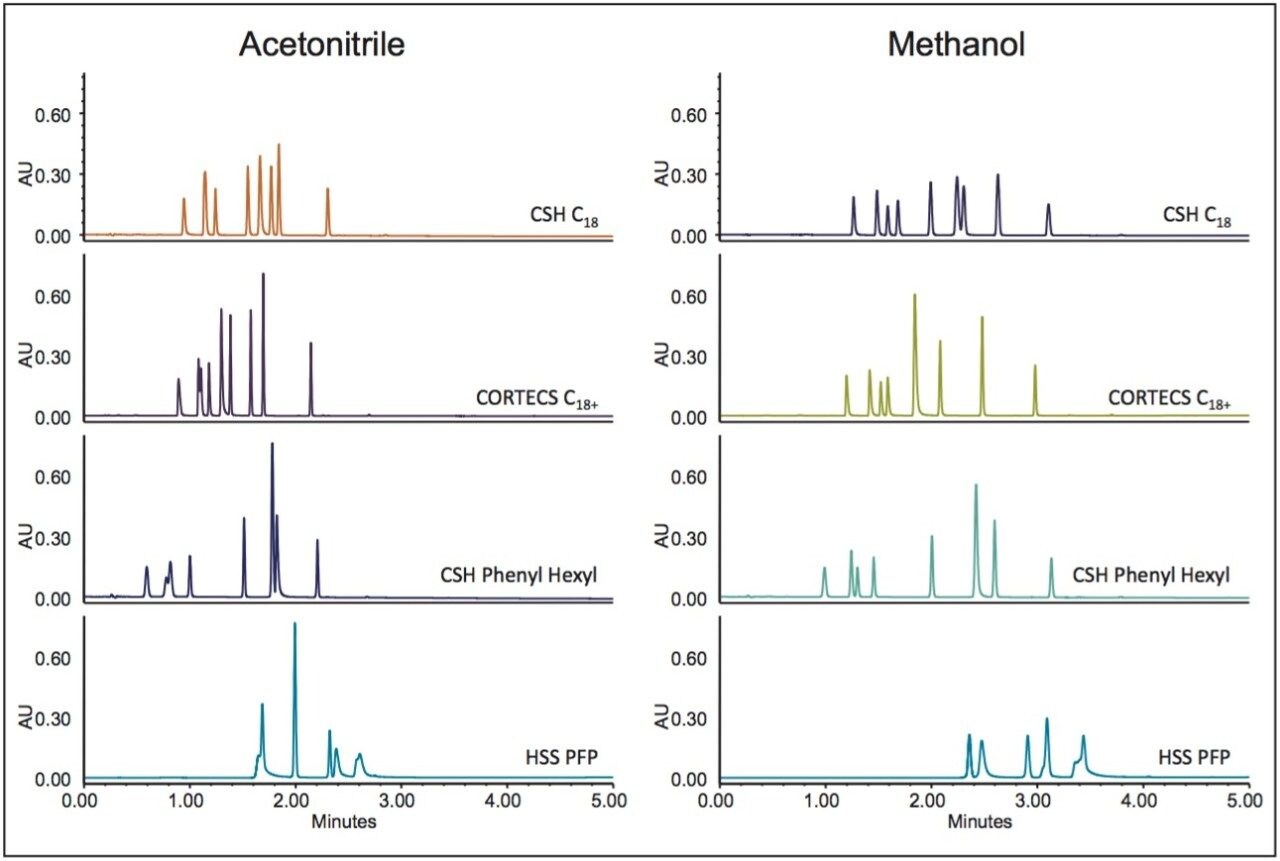
We selected metoclopramide and its USP-defined related substances assay to demonstrate this protocol and to highlight the use of Empower Custom Calculations and Custom Reporting. Shown in Figure 2 are the chromatograms for the screening phase in the protocol. From our previous steps, we had selected a low pH region, and in this phase we were selecting a column chemistry and elution solvent. For each separation, Empower identified each integrated peak and calculated system suitability parameters.
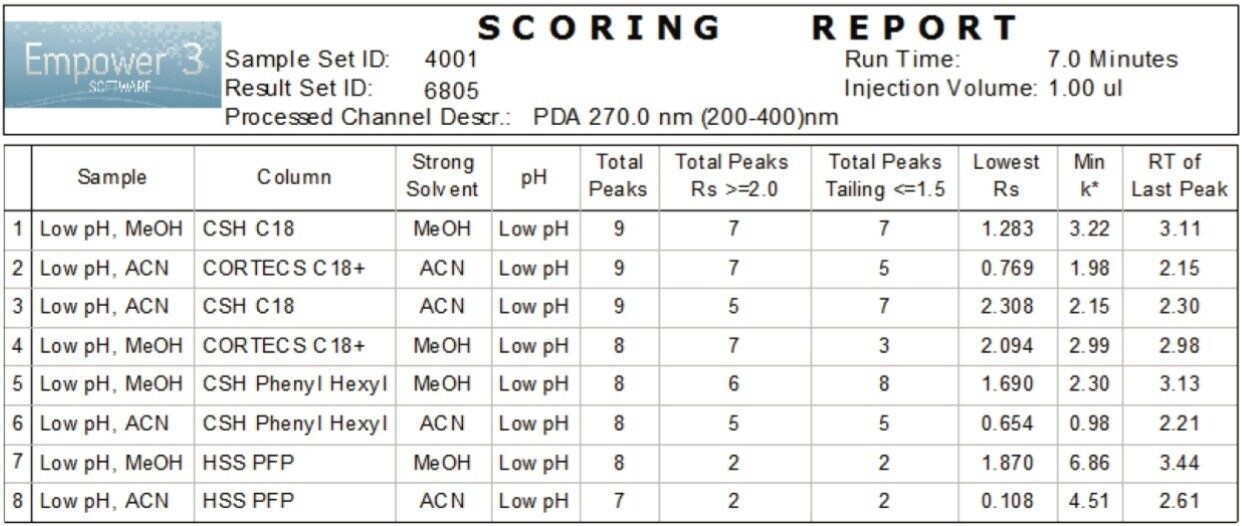
Using Custom Calculations we were able to automatically tally the total number of peaks, the number of peaks with a USP resolution ≥2.0, and the total number of peaks with USP tailing ≤1.5 for each separation. Using a Custom Report, Figure 3, we tabulated and ranked each separation using these criteria. We also included additional important data, such as lowest resolution, k* a measure of retention in reversed-phase chromatography, and the retention time of the last eluting peak. This automated scoring allowed us to quickly identify the conditions that met our criteria and removed analyst variability and bias in decision making in our method development process.
Using Empower 3 Software and its Custom Calculations and Custom Reporting functions enables users to quickly and automatically evaluate chromatographic data. Reports can be configured to allow users to choose optimal conditions based on metrics rather than analyst judgment, minimizing analyst bias and ensuring a comprehensive assessment of data and best method conditions.
720004978, March 2014