This application note demonstrates confirmatory GC-MS/MS method for the analysis of complex PCDDs and PCDFs in foods and feeds that is in compliance with recent EU Regulation 589/2014. The use of APGC in combination with the Xevo TQ-S for the analysis of dioxins has the same potential, in terms of sensitivity and selectivity, as the traditional HRMS instrumentation used for this analysis, and that it is compliant with Regulation 589/2014/EU.
The term dioxins refers to a group of chemically similar congeners, polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs), known to persist in the environment and pose significant toxicological concern. Therefore, they are well regulated and testing is enforced globally. Testing has been traditionally performed by GC-EI-HRMS. However, recent technological advances have allowed for a revision of the analytical criteria. Following extensive review, the European Commission has enacted Regulation 589/2014, which permits the use of GC-MS/MS for the confirmatory analysis of dioxins in food and feed.1
In this application note, we summarize a comprehensive study completed by van Bavel, et al. that demonstrates the capabilities of Waters Atmospheric Pressure Gas Chromatography (APGC), coupled with Xevo TQ-S for the determination of dioxins in a variety of sample matrices. The performance of APGC was compared to samples previously characterized by GC-HRMS, the gold standard in dioxin analysis. Finally, system robustness was investigated by an inter-laboratory comparison trial using four different Xevo TQ-S systems with APGC. A more detailed description of the method and results achieved are described by van Bavel et al.2
|
GC system: |
7890A |
|
Column: |
DB-5MS (60 m x 0.25 mm id. x film thickness 0.25 μm) or BPX-5 (30 m x 0.25 mm I.D. x 0.25 μm) |
|
Injection: |
1 μL pulsed splitless mode (at 280°C) or 5 μL MMI PTV (at 100 °C, 0.5 min; 340 °C, 20 min) |
|
Transfer line temp.: |
280 °C to 360 °C |
|
Carrier gas flow: |
1.4 to 2 mL. min-1 (helium) |
|
Auxiliary gas: |
250 to 300 L. h-1 (nitrogen) |
|
Make-up gas: |
150 to 370 mL. min-1 (nitrogen) |
|
MS system: |
Xevo TQ-S |
|
Corona pin: |
1.8 to 2.1 μA |
|
Source temp.: |
150 °C |
|
Cone gas flow: |
170 to 200 L.h-1 |
|
Collision gas: |
2.5 to 6.2 x 10-3 mbar (argon) |
|
Acquisition: |
MRM mode, as shown in Table 1 |
|
Quantitative analysis was performed in MRM mode |
|
|
Data management: |
MassLynx MS Software, v4.1, with TargetLynx Application Manager |
EPA-1613 CSL to CS5 Standards, containing both native and 13C labelled PCDD, PCDF and TCDD compounds, were used for calibration curves. For method performance and sample preparation the following standards were used: EPA-1613 PAR, EPA-1613 LCS and TF-TCDD-MXB, along with 13C- labelled EPA-1613 ISS PCDD and PCDF congeners. All standards were purchased from Wellington Laboratories (Ontario, Canada). A further dilution of the CSL standard was made in nonane to give a 10 fg.μl-1 standard.
A wide variety of characterized samples were investigated in this study. Certified reference materials of BCR-607 (milk powder), BCR-677 (sewage sludge), and BCR 490 and BCR-615 (fly ash) were acquired from the Institute for Reference Materials and Measurements (IRMM), European Commission Joint Research Centre (Geel, Belgium). Internal reference materials for routine quality control in the four laboratories: human blood and naturally contaminated food and feed samples, international intercomparison studies (fish), and proficiency tests organized by the EU reference laboratory were also used to compare results from different systems. Samples were prepared following previously validated methods, standard methods, or based on analytical criteria of the EU Commission.3-7
A summary of the instrument conditions used across the four different laboratories are provided here. More specific APGC-TQ-S instrument conditions are detailed by Dunstan et al.8
The analysis of dioxins and furans was completed using the Xevo TQ-S with APGC across four different laboratories: 1. Örebro University, Örebro, Sweden; 2. University Jaume I, Castellón and IAEA-CSIC, Barcelona, Spain; 3. EURL for Dioxins and PCCBs in Feed and Food, Freiburg, Germany, and 4. Waters, Manchester, UK. Following instrument optimization, an inter-laboratory comparison was performed using certified reference materials. These results were further compared to those obtained by the traditional GC-HRMS technique.
APGC-MS/MS ionization under charge-transfer conditions (dry source) revealed an abundant presence of the molecular ion for all 17 of the 2,3,7,8 chlorine substituted dioxins and furans. Therefore, MS/MS scans were performed in order to find selective transitions based on the use of M+• as precursor ion.
Collision energies of 30 and 40 eV were selected for all of the PCDDs and PCDFs, respectively, and are summarized in Table 1. At low collision energies, the product ion spectrum was dominated by the 35Cl loss, but at the final optimum collision energies, the transitions selected corresponded to the loss of [CO35Cl]. This fragmentation is very specific for dioxins and furans, providing beneficial selectivity.
Analytical performance was evaluated across four different laboratories investigating detection limit, linearity, ion ratios, repeatability, and reproducibility of the method. For ease of interpretation, these parameters are compared to HRMS requirements presented in EPA1613 or EN 16215 for dioxin analysis.
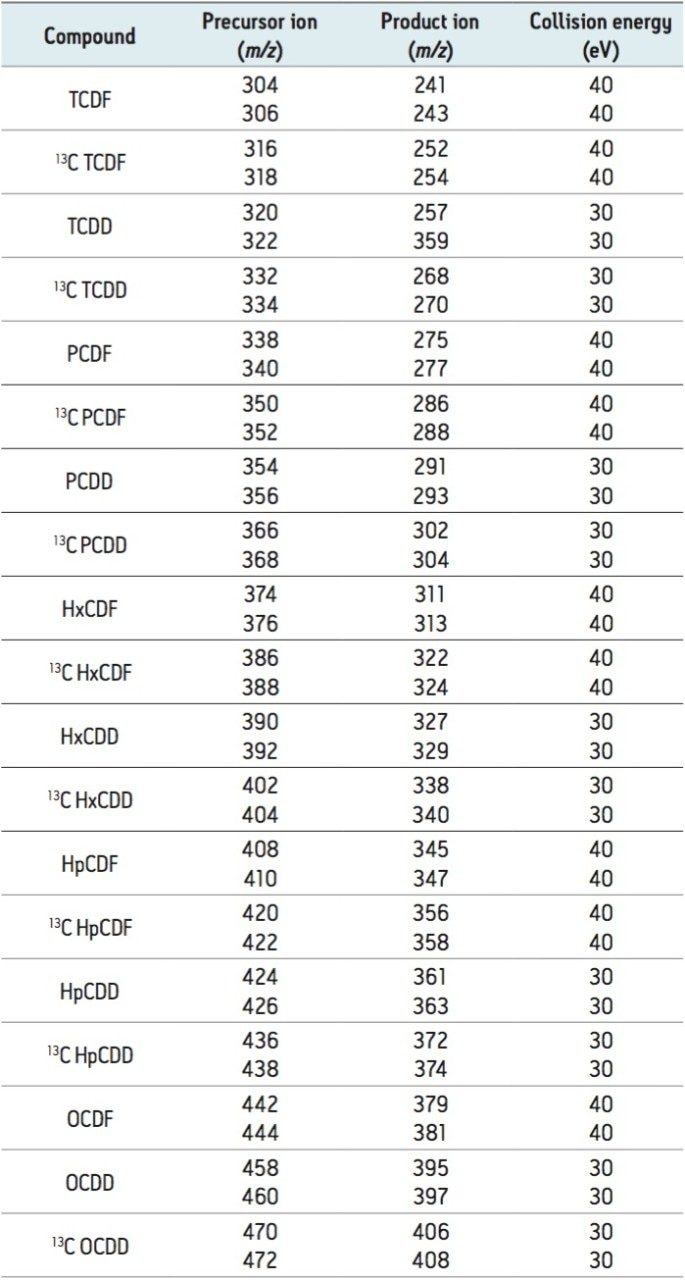
The sensitivity for all tetra- to octa-substituted PCDD/DFs was investigated. TeCDD/TeCDF, PeCDD/PeCDF/HxCDF/ HxCDD/HpCDD/HpCDF, and OCDD/OCDF were analyzed at 10, 50, and 100 fg.μl,-1 respectively, as shown in Figure 1.
Typically for the evaluation of high resolution mass spectrometry instruments a 100 fg.μl-1 standard of 2,3,7,8-TeCDD is monitored, where a signal-to noise (S/N) ratio of >100 is required. Therefore, following the initial setup of the APGC-Xevo TQ-S, the lowest calibration point for 2,3,7,8-TeCDD in this method was diluted to 10 fg.μl.-1 This solution readily achieved a S/N ratio of >50 in all four laboratories, well below the required limit.
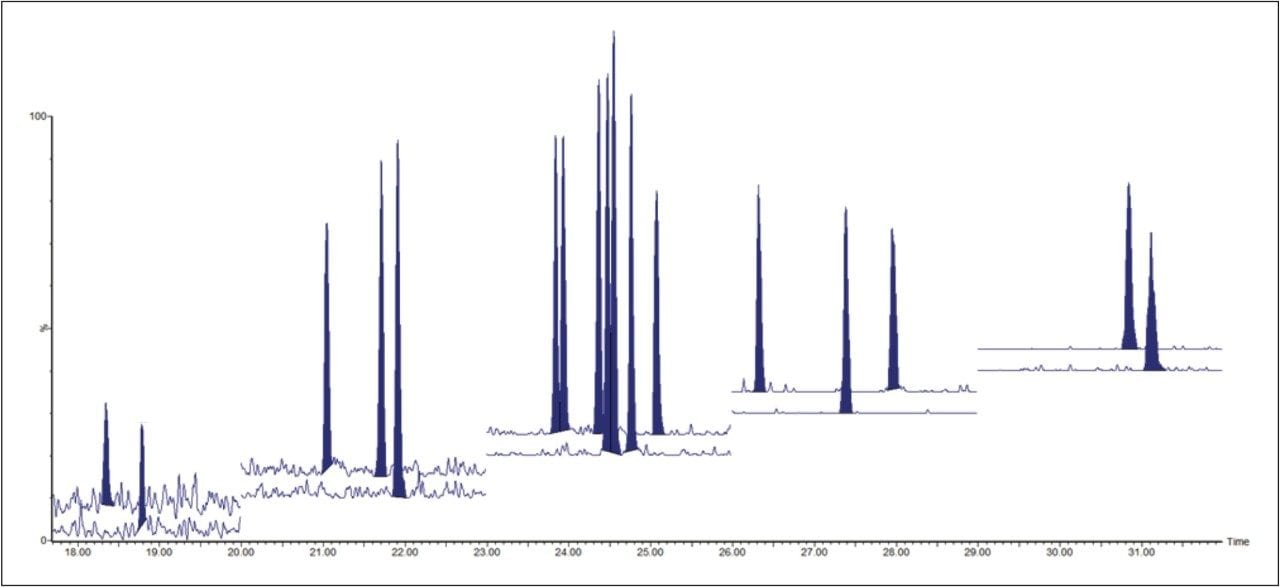
The ultimate sensitivity was tested using a mixture of TCDD congeners (TF-TCDD-MXB) at concentrations of 2, 5, 10, 25, 50, and 100 fg.μl,-1 where 2 fg.μl-1 on column allowed for satisfactory detection of the congeners, as shown in Figure 2. All of these results are impressive and in good agreement with, or even better than those routinely achieved with high resolution magnetic sector GC-MS systems.
The linearity of the method was studied by analyzing the standard solutions (in triplicate) at six concentrations, ranging from 0.1 to 40 pg.μl-1 (EPA-1613 CSL to CS4) on the four different systems. The linearity, using internal standard calibration, was satisfactory with coefficient of determination (R2) >0.998. The relative standard deviation (RSD) of the relative response factors (RRFs), as defined in standard methods EPA 1613 or EU 1948, was also achieved (i.e. below 15%), as required by both methods. Based on area, the repeatability was within 15% for the injection of 10 fg.μl-1 (n=3-10), and below 10% for all PCDD/DFs for the CSL standard against the corresponding 13C standard (RRF).
An important criterion for the unequivocal identification of the PCDD/F congeners is the ion abundance ratio between the two monitored product ions, resulting from two different precursor ions. For quality control, the ion abundance ratios can be compared with calculated or measured values. The calculated ratio depends on the relative abundance of the two selected precursor ions ([M+• 35Cl] and [M+• 37Cl]), and their relative loss of [CO35Cl] or [CO37Cl] that result in the formation of each product ion. It is only comparable with the measured ratios, if identical collision energy and collision gas pressure is applied for both transitions. The measured ion abundance ratios in the sample extracts matched those of the calibration standards within the QC limits of ± 15%, as derived from EPA 1613 for HRMS and EU Regulation 589/2014.
The ion abundance ratios, in combination with the relative response factors from the calibration curves can further be used to check the reliability of the results in the low concentration range. Limits of quantification (LOQs) were determined based on maximum deviations (±15%) of the calculated value for ion abundance ratios and deviations (≤30%) of the relative response factor of the mean value. With an RSD of ≤20% for the complete calibration, LOQs in the range of 10 to 30 fg (on column) were obtained for 2,3,7,8-TCDD and 2,3,7,8-TCDF.
In order to test the capabilities of the developed method, proficiency samples outlined in the experimental section were previously run and characterized on high resolution systems. These samples were re-injected on the APGC-MS/MS system in three different laboratories: the EURL for Dioxins and PCBs in Germany, CSIC and IUPA in Spain, and MTM in Sweden. Each laboratory tested different samples by GC-HRMS and APGC-MS/MS, as shown in Figure 3. Excellent correlation between the instruments was demonstrated, where the relative difference between the APGC and the HRMS results <7% for all samples. Like HRMS, the APGC runs passed all QA/QC criteria in terms of chromatographic separation, linearity, S/N ratio, and ion abundance ratio of selected transitions.
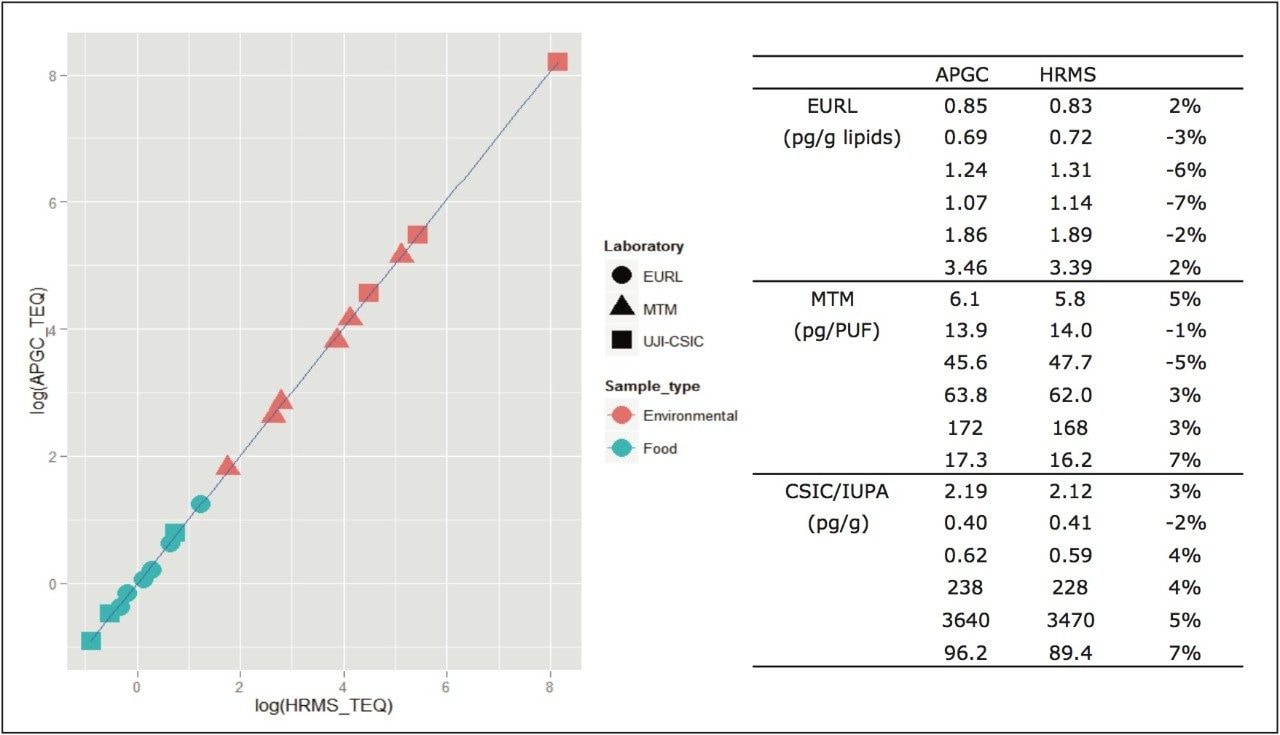
An additional summary of quality control food samples analysed by both GC-HRMS and APGC-TQ-S is given in Figure 4. Excellent correlation is evident between the two techniques, over a wide concentration range and in a variety of food matrices.
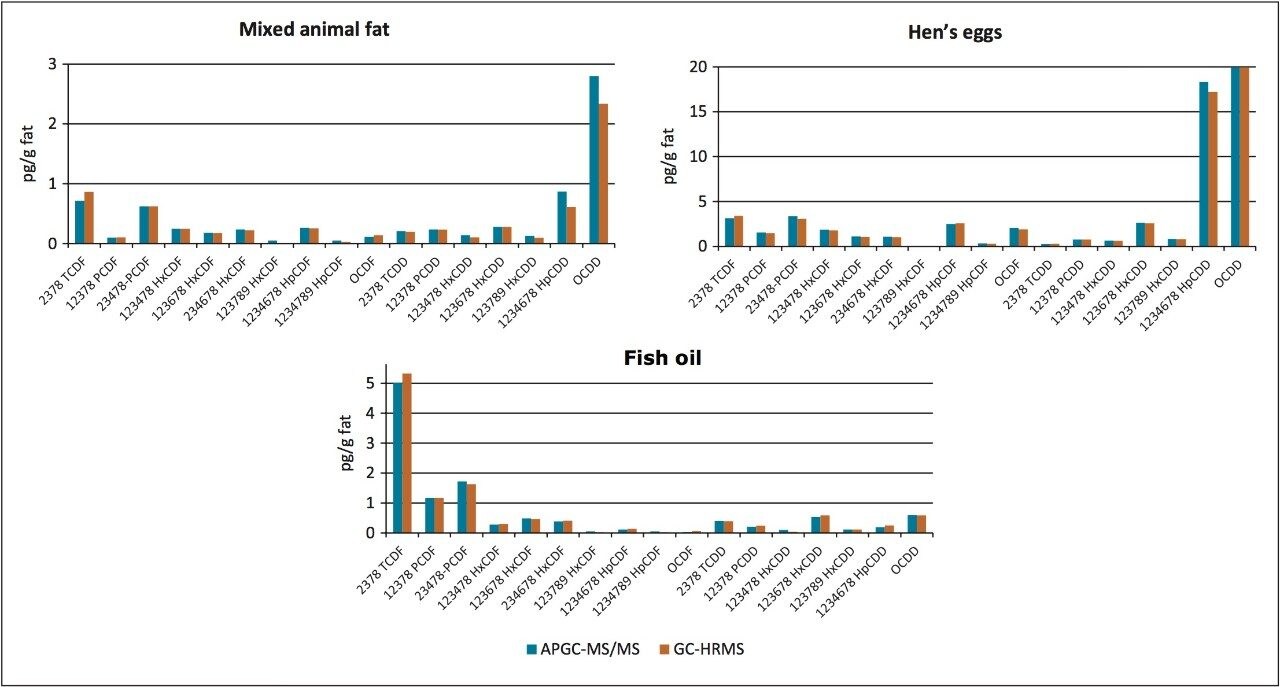
The potential of using APGC-TQ-S for the identification and quantification of dioxins and furans in a variety of complex matrices has been successfully demonstrated. Following the recent change to analytical criteria in EU Regulation 589/2014, GC-MS/MS can now be used as a confirmatory method for the analysis of PCDDs and PCDFs in foods and feeds.
Van Bavel, et al. have demonstrated that the results of the APGC system are impressive and comparable with HRMS not only in selectivity but also in sensitivity.2 Excellent linearity was achieved (R2 >0.998) over an appropriate calibration range.
The results from a wide variety of complex samples previously analyzed by GC-(EI)-HRMS were compared with the results from the APGC-MS/MS system. Results between instruments showed excellent agreement, both in terms of the individual congeners and toxic equivalence factors (TEQs).
The authors conclude that the use of APGC in combination with the Xevo TQ-S for the analysis of dioxins has the same potential, in terms of sensitivity and selectivity, as the traditional HRMS instrumentation used for this analysis, and that it is compliant with Regulation 589/2014/EU. The APGC-MS/MS benchtop system, however, is far easier to use, maintain, and it can be quickly converted for liquid chromatography analysis.
720005219, November 2015