In this application note, we have demonstrated the ability of an ACQUITY UPLC system combined with an ACQUITY UPLC PDA and SQ mass spectrometer to separate degradation products in a pharmaceutical product, such as simvastatin. These high peak capacity separations for complex mixtures of degradation products result in faster analyses, improve identification of impurity products and shorten the time required to develop stability indicating methods, improving the quality and throughput of forced degradation studies.
Chemical stability is one of the most important issues that impacts the quality and safety of a pharmaceutical product. The FDA and ICH require stability testing data to understand how the quality of an API or a drug product changes with time under the influence of environmental factors such as heat, light, and humidity.1,2 Knowing the stability characteristics of a pharmaceutical allows for the establishment of storage conditions and shelf life, the selection of proper formulations and protective packaging, and is required for regulatory documentation.
Forced degradation, or stress testing, is similar to stability testing but carried out under harsher conditions than those used for accelerated testing. Forced degradation is generally performed early in the drug development process and is the main tool used to predict stability related properties, to understand degradation products and pathways and to develop stability indicating methods.3
The most common analytical technique for monitoring forced degradation experiments is HPLC with UV and/or MS detection, allowing for peak purity, mass balance, and identification of degradation products. These methodologies are often time consuming and of medium resolution, requiring analysis times of 30 minutes or more4 to ensure that all of the degradation products are accurately detected. The use of UltraPerformance LC (UPLC)/UV/MS allows for faster and higher peak capacity separations, which can aid in the analysis and identification of degradation products and shorten the time required to develop stability indicating methods. The purpose of this application note is to demonstrate the advantages of resolution and sensitivity that UPLC brings to forced degradation studies.

Forced degradation studies were carried out on simvastatin under varied conditions of acid/base hydrolysis, thermal degradation, peroxide oxidation, and photo degradation, with the ultimate goal of achieving 10 to 20% degradation (loss of API). Additional degradation products to those normally observed in real time or accelerated stability testing were generated.
Acid and base hydrolysis and peroxide degradation were carried out on simvastatin in solution (10 mM ammonium acetate, pH 4.5) while thermal degradation was performed on simvastatin solid. Photostability measurements were performed on both simvastatin solid and in solution. Solution degradation experiments were carried out at a simvastatin concentration of 1 mg/mL. The degraded samples were diluted to a concentration of ~0.1 mg/mL (1:10 dilution) prior to injection on the UPLC/UV/MS system. The data generated was used to monitor the effects of the stress conditions on the simvastatin.
|
LC system: |
Waters ACQUITY UPLC system |
|
LC data software: |
Waters Empower 2 CDS software |
|
Column: |
ACQUITY UPLC BEH C18 column 2.1 x 50 mm, 1.7 μm |
|
Column temp.: |
45 °C |
|
Flow rate: |
600 μL/min |
|
Mobile phase A: |
10 mM ammonium acetate, pH 4.5 |
|
Mobile phase B: |
Acetonitrile |
|
Gradient: |
Linear gradient 25 to 90% B over 7 min |
|
MS system: |
Waters SQ mass spectrometer |
|
Ionization mode: |
ESI positive |
|
Capillary voltage: |
3200 V |
|
Cone voltage: |
20 V |
|
Desolvation temp.: |
350 °C |
|
Desolvation gas: |
900 L/Hr |
|
Cone gas: |
50 L/Hr |
|
Source temp.: |
130 °C |
|
Acquisition range: |
100 to 900 m/z (5000 Da/sec) |
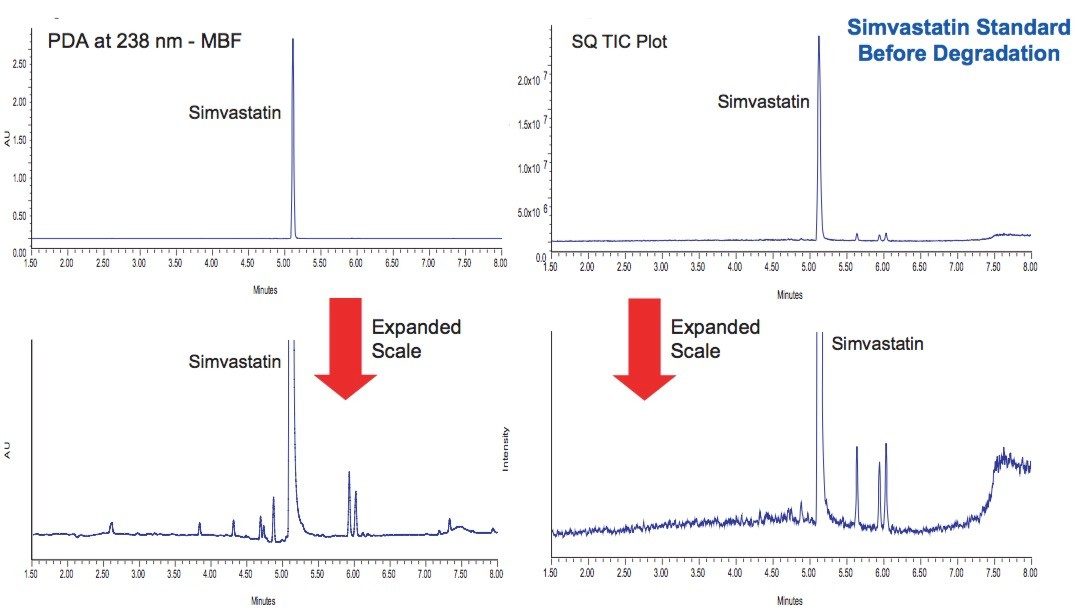
A standard solution of simvastatin was injected at a concentration of 0.1 mg/mL as a control (Figure 2). The major peaks were identified and confirmed by orthogonal acceleration time-of-flight (oa-TOF) MS to be the commonly observed impurities often found in simvastatin. This chromatographic data is the baseline that will be used to evaluate results of stress testing studies.
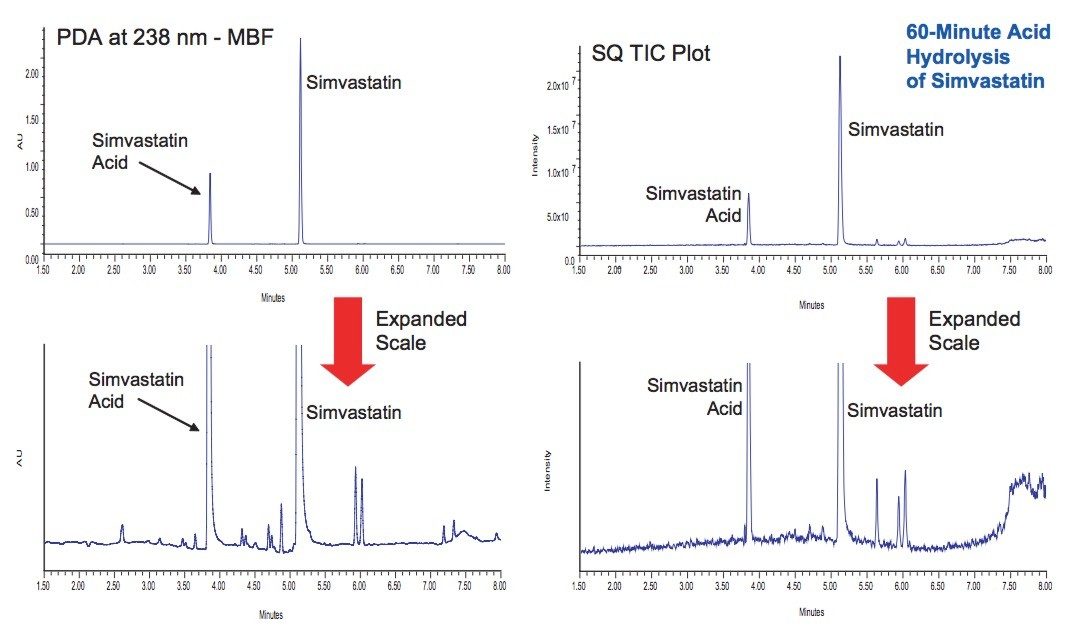
UPLC/UV/MS analysis of the acid and base hydrolysis of simvastatin showed it to be extremely sensitive to pH (Figure 3). Above pH 8, simvastatin rapidly undergoes hydrolysis to be completely converted to simvastatin acid, which agrees with previously published work.5 These studies demonstrated that hydrolysis of simvastatin in 100 mM hydrochloric acid for 1 hour and 15 mM sodium hydroxide for 45 minutes both resulted in the desired 10 to 20% loss of the initial API.
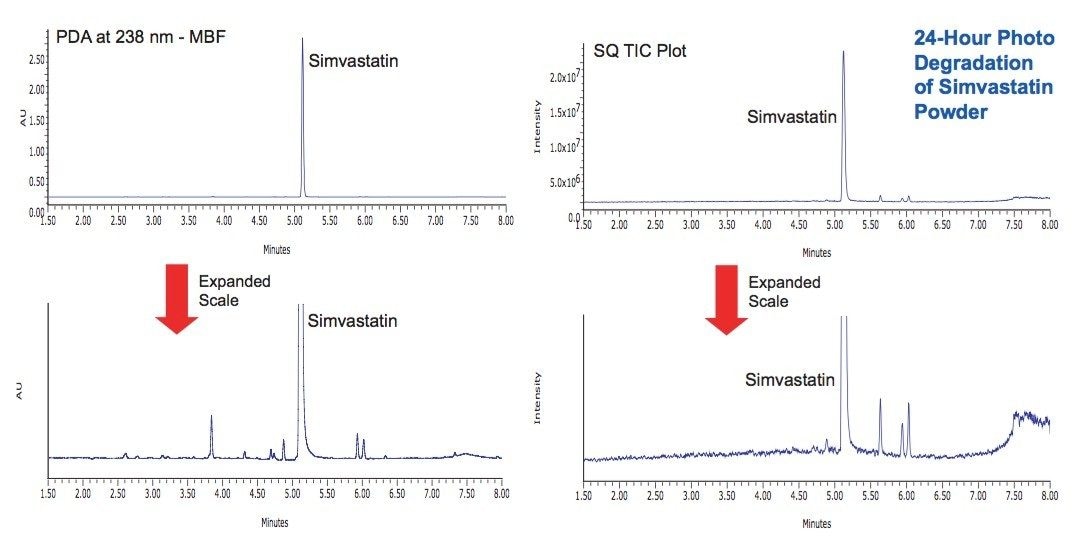
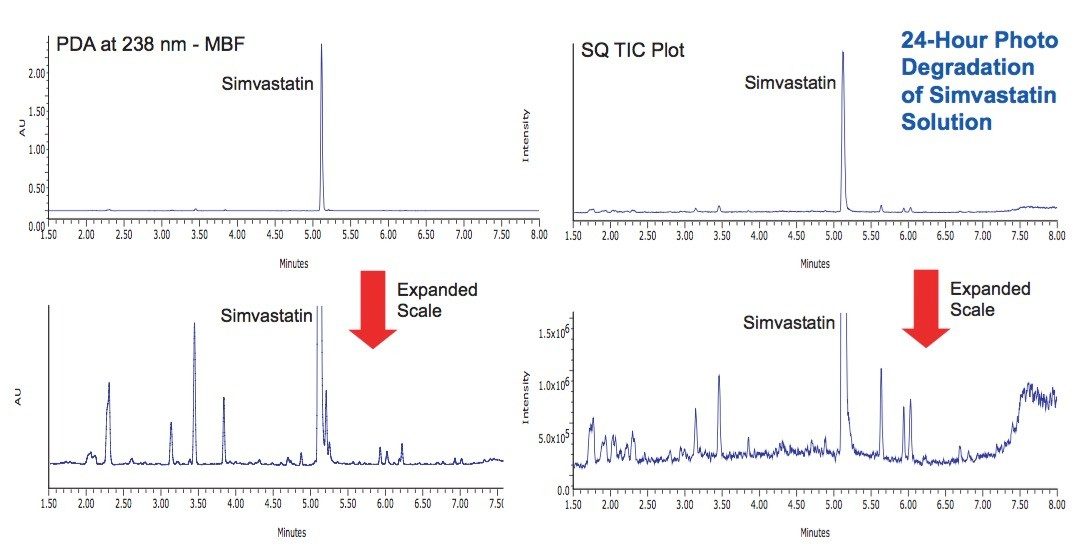
Photostability studies were performed on simvastatin both as a dry powder and in solution (1 mg/mL in 10 mM ammonium acetate, pH 4.5). Samples were exposed for 8 hours and 24 hours at the maximum Suntest CPS lamp intensity (583 and 1750 Watt Hrs/ m2, 320 to 400 nm). In the solid state, simvastatin was observed to be very stable with little evidence of degradation (Figure 4). In solution, the simvastatin exhibited significant degradation after 24 hours, and a unique profile of degradation products was observed (Figure 5).
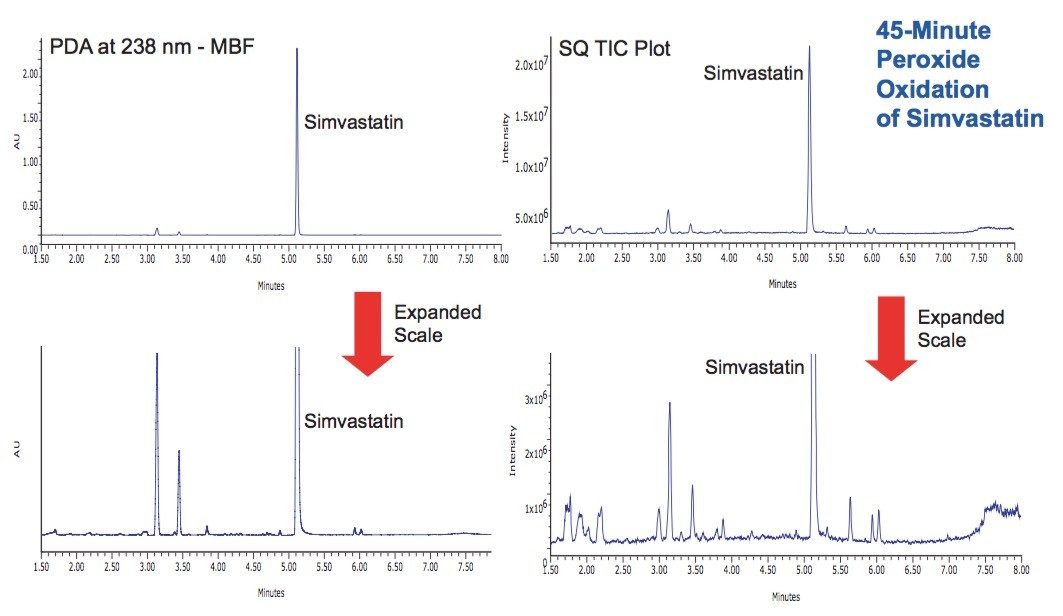
Oxidative degradation is most commonly achieved using peroxides, metal ions (metal salts), or radical initiators such as AIBN (autoxidation). This study found than a 7.5% hydrogen peroxide solution at 55 °C for 45 minutes was sufficient to degrade the simvastatin by ~15% of intitial concentration (Figure 6), resulting in many more degradation products than observed by simple acid or base hydrolysis.
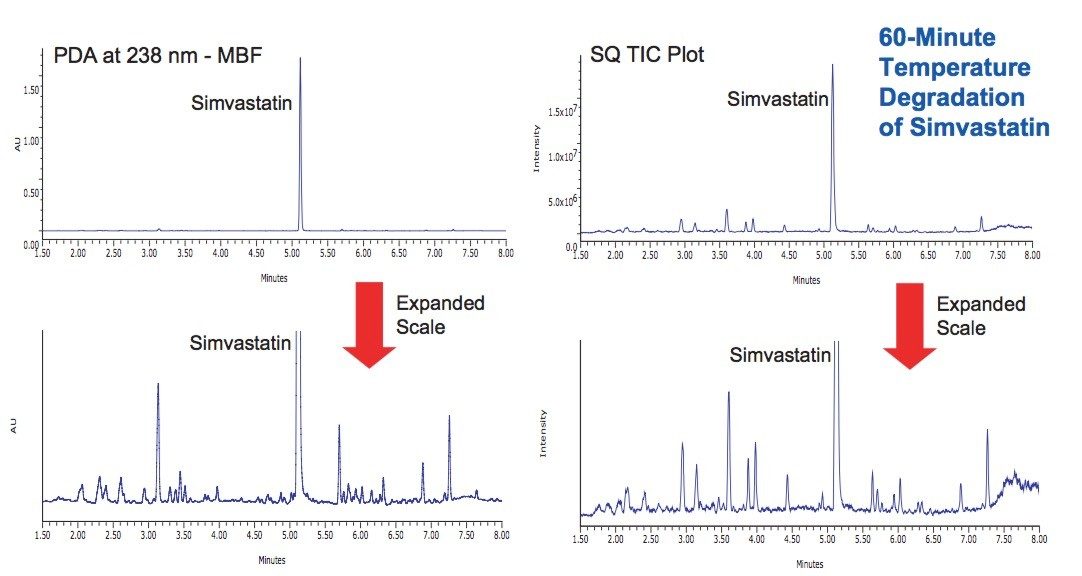
Simvastatin was thermally stressed as a dry powder at 115 °C. After 60 minutes the starting material was degraded sufficiently to detect a number of degradation products. Many of the same degradation products observed from the peroxide oxidation study were also present in the temperature degradation sample (Figure 7).
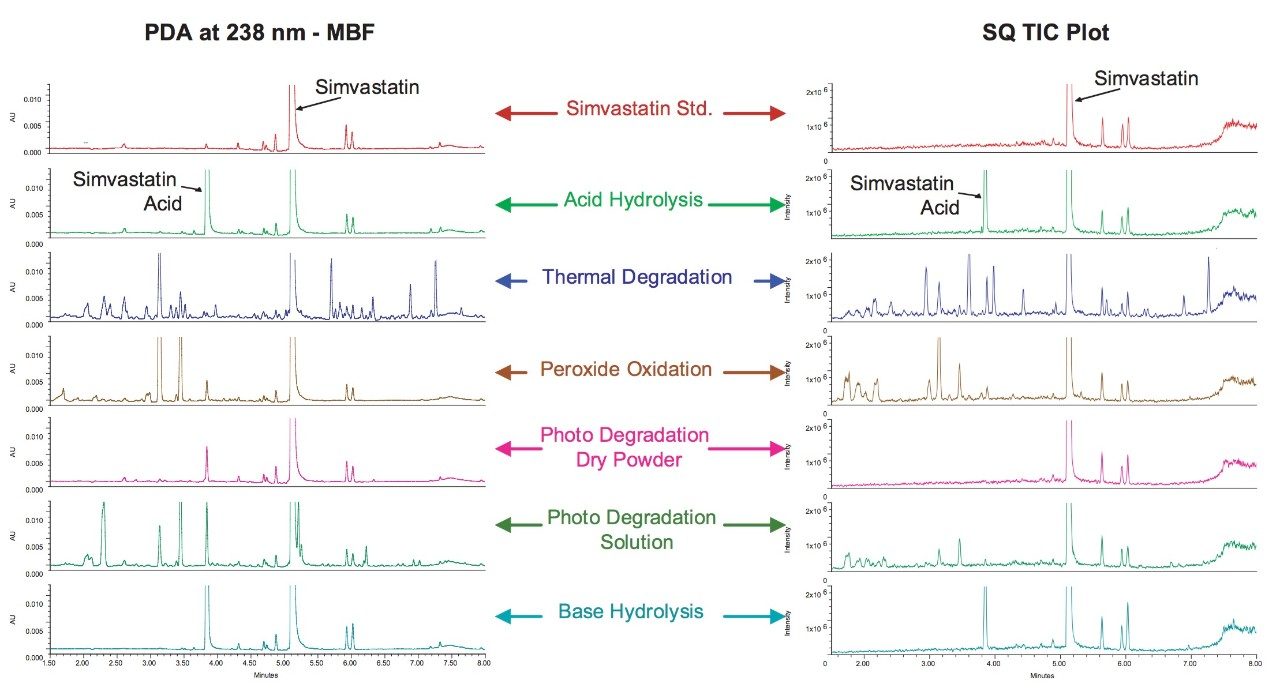
The comparison of chromatograms obtained from analysis of the forced degradation of simvastatin by acid and base hydrolysis, thermal degradation, peroxide oxidation, and photo degradation demonstrate the varied degradation product profiles that result from these various procedures (Figure 8). Although acid or base hydrolysis yields only simvastatin acid as a degradation product, other procedures such as photo and thermal degradation produce much more complicated and unique profiles of degradation products. The high efficiency separations obtained with ACQUITY UPLC systems allow for the easy analysis of these complex mixtures.
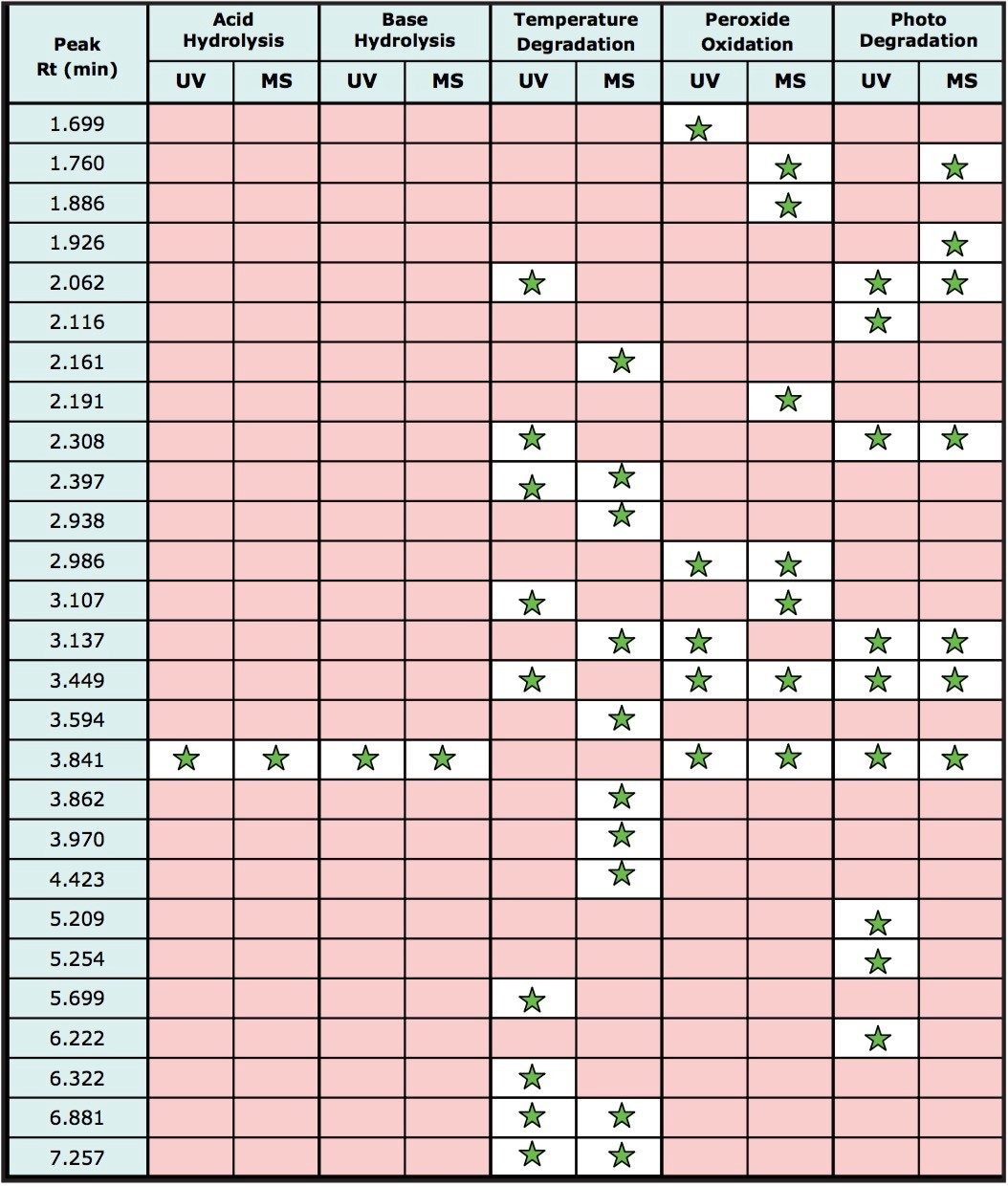
Figure 8 also demonstrates the utility of the combined detection of photodiode array and MS detection. Many of the degradation products were observed in the MS data channel only as the components do not contain chromophores thus are not detected by UV absorption. For other degradation products, UV detection was determined to be more sensitive than MS detection, this is mainly due to the lack of ionizable groups on the molecules. Table 1 lists the major degradation peaks observed for each degradation method including which type of detection was able to detect the degradation products. A multi-detector approach is clearly desirable in order to detect the maximum number of degradation products.
In this application note, we have demonstrated the ability of an ACQUITY UPLC system combined with an ACQUITY UPLC PDA and SQ mass spectrometer to separate degradation products in a pharmaceutical product, such as simvastatin. These high peak capacity separations for complex mixtures of degradation products result in faster analyses, improve identification of impurity products and shorten the time required to develop stability indicating methods, improving the quality and throughput of forced degradation studies.
720002259, July 2015