
This is an Application Brief and does not contain a detailed Experimental section.
This technology brief demonstrates the use of Empower 3 MS Peak Tracking in the tracking of peak elution during development of a UPLC method for metoclopramide HCl and related substances by mass detection.
Utilizing the Empower 3 MS Peak Tracking enables users to correctly track retention time of each component in a sample mixture by mass detection. Empower can quickly display results generated from multiple experimental runs in a report to show retention time of each peak summarized by the mass-to-charge ratio. This streamlines the entire method development processes by accurately tracking selectivity changes of each component over different experiments. Combining UV with MS increases the efficiency of developing robust and reliable methods, and improving overall laboratory throughput.
Method development typically involves screening a range of chromatographic parameters such as columns, organic solvents, buffers, gradient slope, flow rate, temperature, and other variables.
Columns with different stationary phases can often alter the selectivity of compounds. Thus, it is necessary to track peaks between method development runs in order to ensure correct peak elution and identity. Incorrect peak tracking can result in incorrect peak identification and prolonged development times, decreasing laboratory efficiency and productivity. In addition, incorrect identification or failure to identify impurities may compromise safety of the pharmaceutical product. For related species, UV tracking requires separate injections using pure standards, which decrease the efficiency of a laboratory's workflow.
Using mass spectra data for peak tracking enables the analytical laboratory to correctly monitor peak retention during method development experiments.
In this study, we will take advantage of mass spectra data acquired using the ACQUITY QDa Detector to identify each peak by mass detection. A new feature of Empower 3 – called MS Peak Tracking – will be used to monitor elution of a metoclopramide-active pharmaceutical ingredient (API) and its USP-specified related substances over a series of column screening experiments.
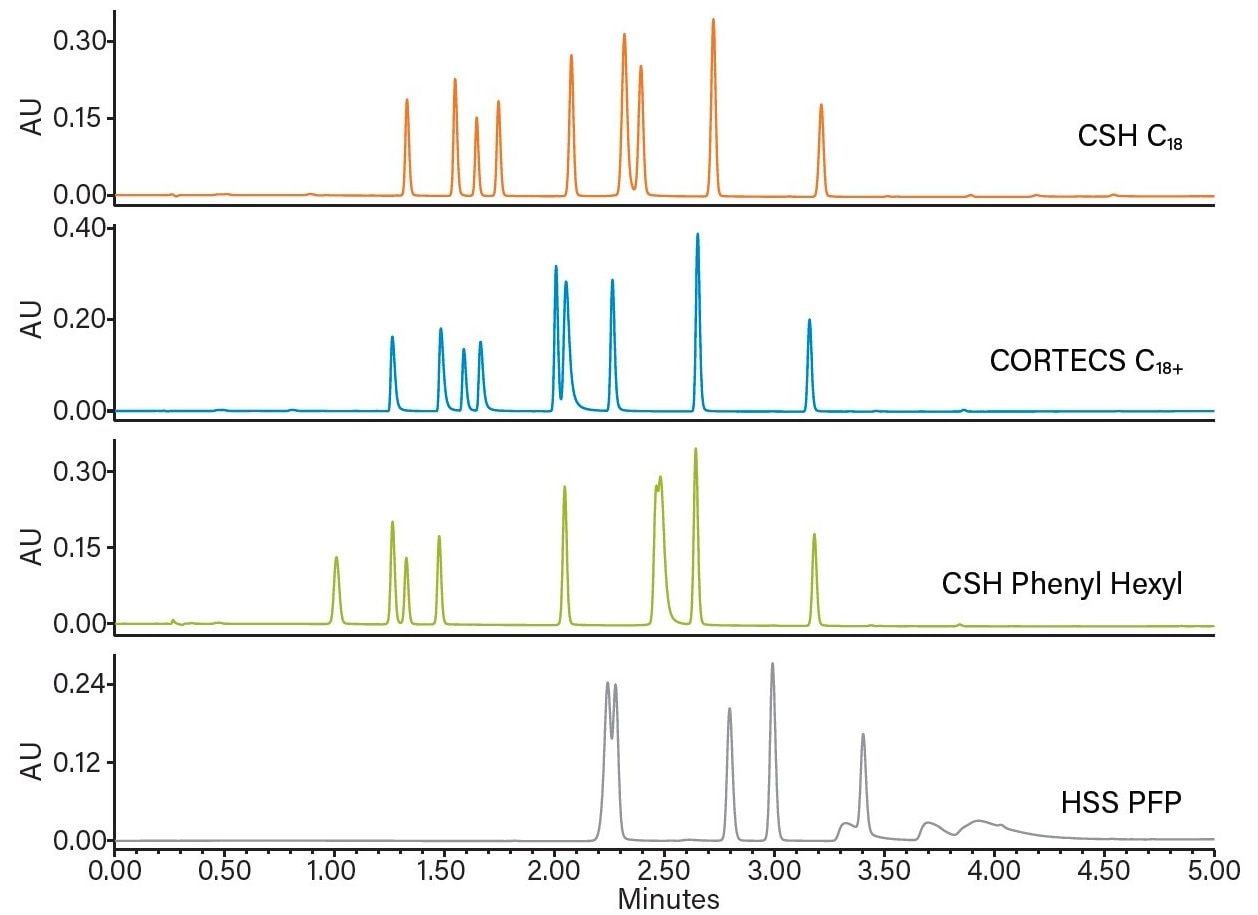
Empower MS Peak Tracking uses the mass-to-charge (m/z) ratio to automatically track each peak in a sample injection.
To illustrate this feature, a systematic screening protocol was used for the development of a UPLC method for metoclopramide and its USP-specified related substances.¹ The protocol includes scouting, screening, and optimization steps, which address factors of retentivity and selectivity to ensure development of robust and reproducible methods.
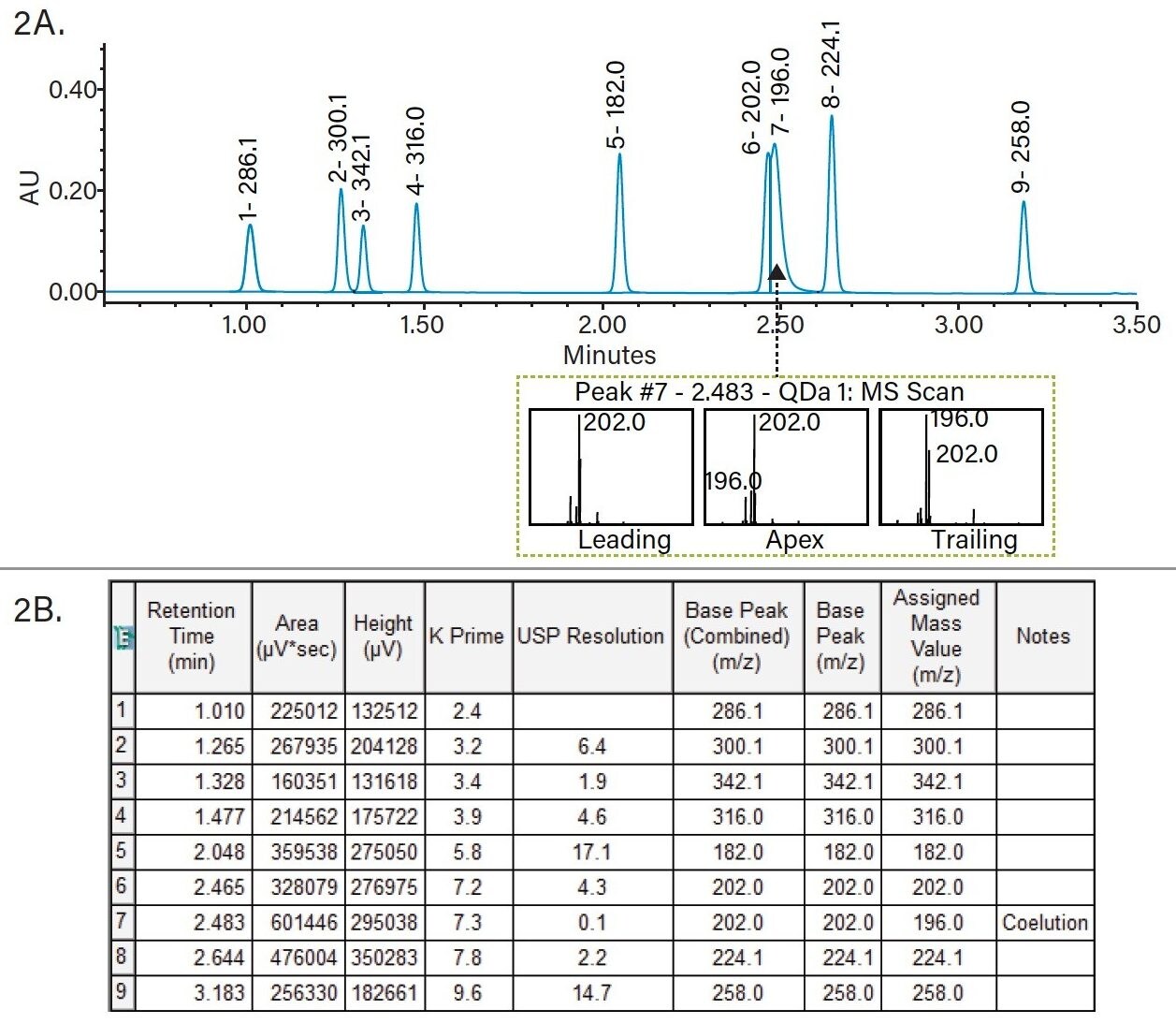
First, we selected the mass spectra for an assigned mass value in the Empower processing method. In this example, we selected apex for an assigned mass and one decimal place for the assigned mass precision. Using these settings we processed the chromatographic data for the column screening experiments (Figure 1). Empower automatically assigned a mass value for each observed peak in the sample. Due to potential challenges of coelution, the assigned mass can be manually changed to a new value. As shown in Figure 2A, data for a sample mixture of metoclopramide and its impurities results in a partial coelution of peaks 6 and 7. Using the mass analysis window in Empower, we can easily view the mass spectral data at the leading, apex, and tailing end of peak 7. As displayed in Figure 2A, there are two masses present – m/z 202.0 and 196.0 – which are specific for impurities C and H, respectively. The intensity of m/z 196.0 is highest at the tailing edge of peak 7, indicating that it corresponds to impurity H. We manually assigned a new mass to that peak by adding a new value and a note with reasoning for the change (Figure 2B). Finally, we used Empower report to display results for the column screening phase. As shown in an Empower report for MS Peak Tracking over multiple chromatographic runs (Figure 3), retention time of each peak is summarized by the assigned mass.
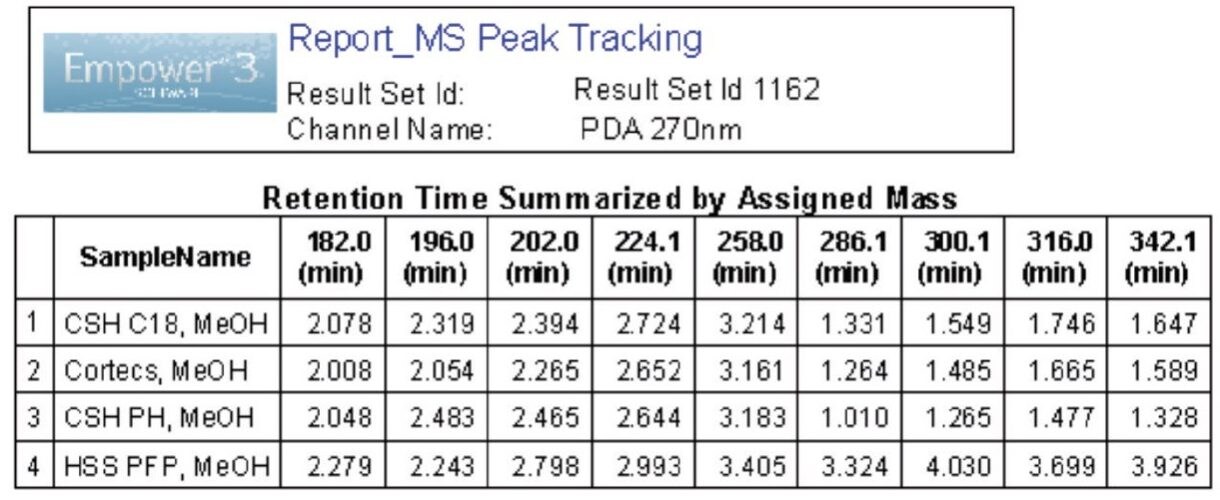
Utilizing the Empower 3 MS Peak Tracking enables users to correctly track retention time of each component in a sample mixture by mass detection. Empower can quickly display results generated from multiple experimental runs in a report to show retention time of each peak summarized by the mass-to-charge ratio. This streamlines the entire method development processes by accurately tracking selectivity changes of each component over different experiments. Combining UV with MS increases the efficiency of developing robust and reliable methods, and improving overall laboratory throughput.
720005721, June 2016