
This is an Application Brief and does not contain a detailed Experimental section.
Utilizing APGC coupled with the Xevo TQ-XS allows sub-femtogram levels of dioxins to be analyzed in complex samples. The added sensitivity enables the dilution of expensive dioxin standards, reduces the need to preconcentrate sample extracts (prior to analysis), and minimizes the amount of sample required for testing.
Not only is this system exceptionally sensitive, it is also robust and produces consistent results over thousands of injections. The APGC coupled with the Xevo TQ-XS far surpasses the regulatory requirements for dioxin testing.
Polychlorinated dibenzo-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs) are a group of chemically related compounds that are toxic and persistent organic pollutants (POPs). These compounds are restricted internationally under the Stockholm Convention¹ and due to the bioaccumlative nature of these compounds, it is essential to monitor them at ultra trace levels in food and environmental samples. Traditionally these compounds have been analyzed using magnetic sectors with electron ionization sources which require expert users to obtain consistent results. As there is a growing concern for the analysis of these compounds, more user-friendly technology is essential to analyze potentially contaminated samples. Atmospheric Pressure Gas Chromatography (APGC), coupled with a highly sensitive tandem quadrupole mass spectrometer (Xevo TQ-S), has already been demonstrated to be a sensitive and robust option for confirmatory analysis of PCDDs and PCDFs by GC-MS/MS in compliance with 589/2014/EU.² The recent introduction of the Xevo TQ-XS from Waters has allowed lower limits of detection to be reached. This can help reduce time spent on sample preparation/preconcentration, as well as reducing the cost of analysis as diluted standards can be utilized.
Agilent DB-5MS column, 30 m x 0.25 mm I.D. x 0.25 µm film, helium at 1 mL/min. 7890A GC Oven and Agilent autosampler, split/splitless injector at 290 °C operating in pulsed splitless mode (32 psi for 0.5 min) with a 1.0 µL injection volume. GC program, initial temp. of 130 °C, hold for 1.2 min, ramp at 20 °C/min to 320 °C, and hold for 3.3 min.
Zebron ZB-5MS column, 60 m x 0.25 mm I.D. x 0.25 µm film, helium at 1.4 mL/min. 7890A GC oven and Agilent autosampler, split/splitless injector at 290 °C, operating in pulsed splitless mode (50 psi for 1.8 min) with a 1.0 µL injection volume. GC program: initial temp. of 130 °C, hold for 1.8 min, ramp at 40 °C/min to 200 °C; ramp 2 at 2 °C/min to 235 °C; ramp 3 at 3 °C/minute to 305 °C; ramp 4 at 20 °C/min to 320 °C, and hold for 5 min. Total run time of 49.85 min.
Corona pin at 2.0 µA, cone gas 260 L/hr, auxiliary gas 200 L/hr, makeup gas 300 mL/min, quad resolutions at 0.7 Da.
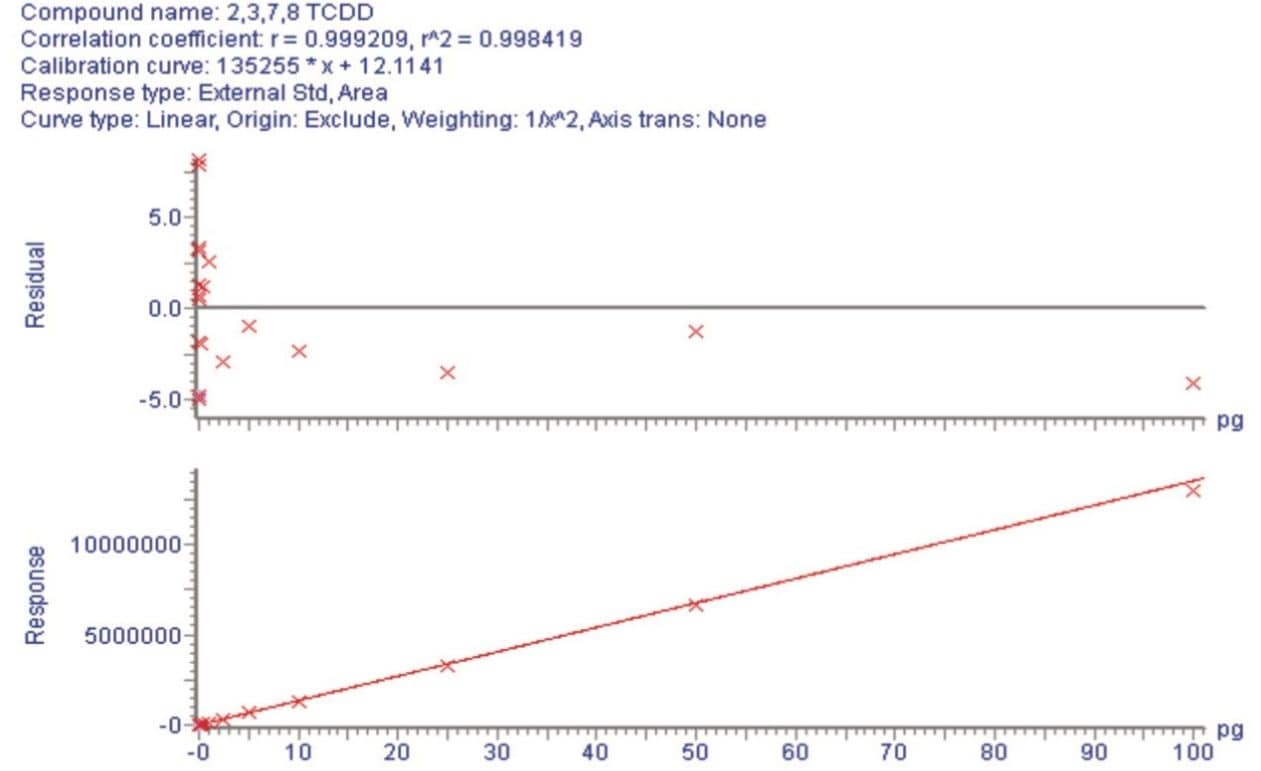
In order to assess the sensitivity of the APGC coupled with the Xevo TQ-XS, a standard of 2,3,7,8-TCDD was diluted in nonane giving a calibration range between 100 ag to 100 pg on column. In order to perform this test, two MRM transitions for TCDD were utilized. Figure 2 shows the linearity of 2,3,7,8 TCDD, which was excellent.
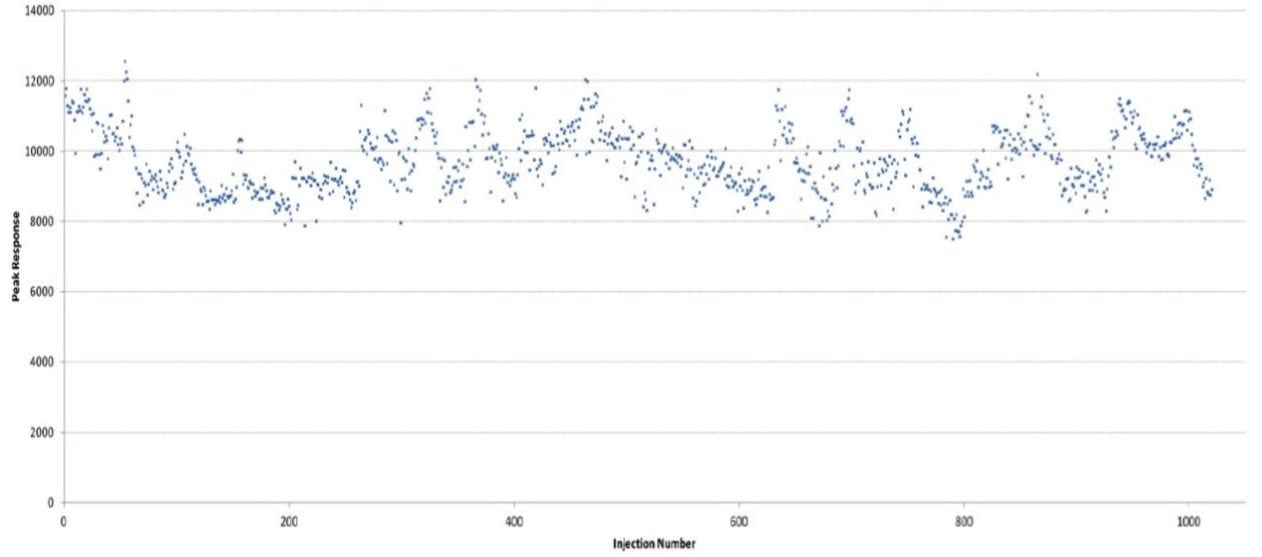
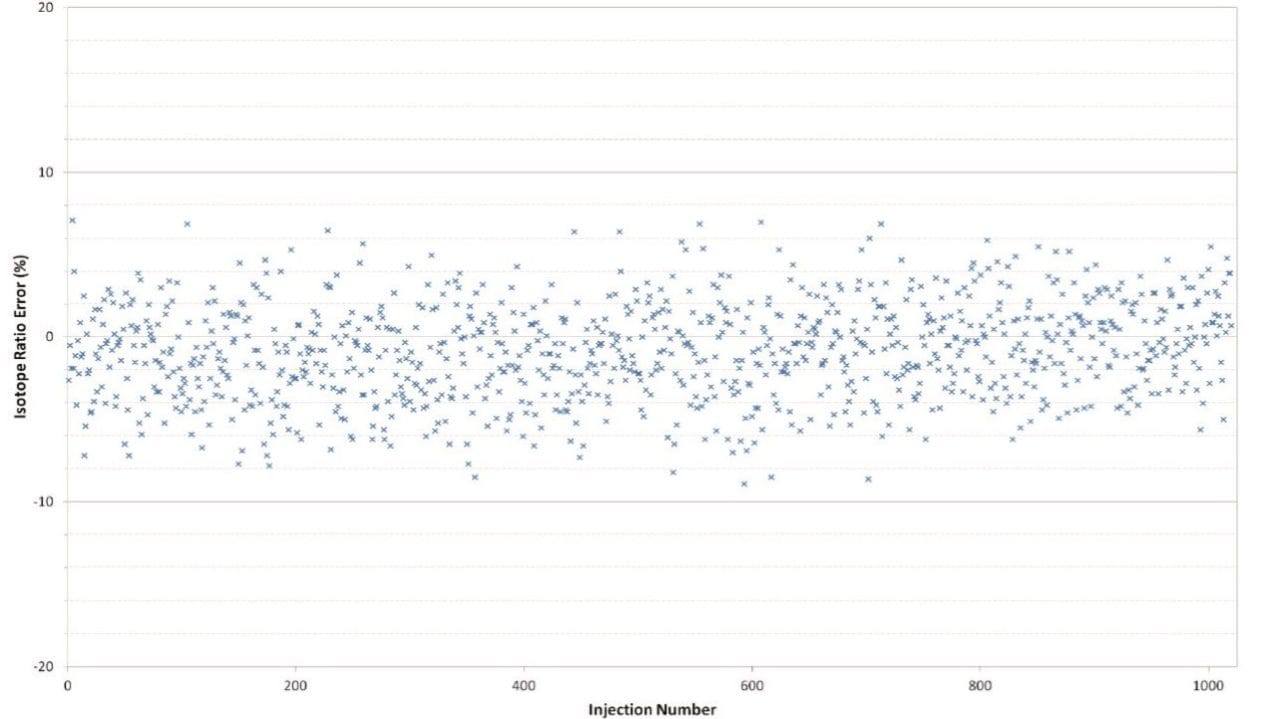
An on-column standard concentration of 100 fg was injected over 20 days in order to assess the reproducibility of the system. Figure 3 shows the outstanding reproducibility of the response, and Figure 4 shows the stability of the isotopic measurements over this series of injections.
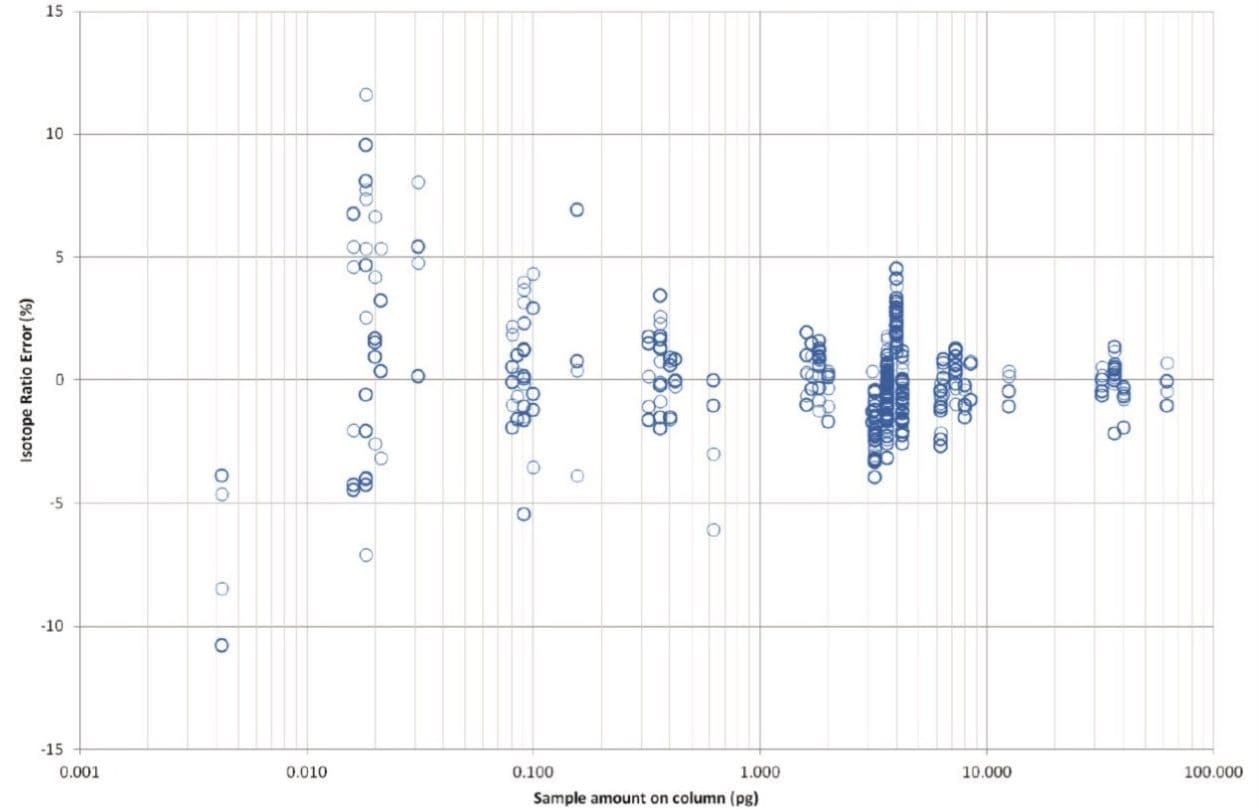
Once the initial sensitivity of the system had been verified, a full suite of TCDDs and TCDFs was acquired on the system. A series of EPA 1613 standards were used from CSL to CS5, diluted 1 in 10 with nonane. Figure 5 shows that the isotope ratio assessment for each congener was consistent at all concentrations. This is essential for the confirmation of dioxins and furans in a sample. Legislation states that these ratios are required to be <15%.3,4,5
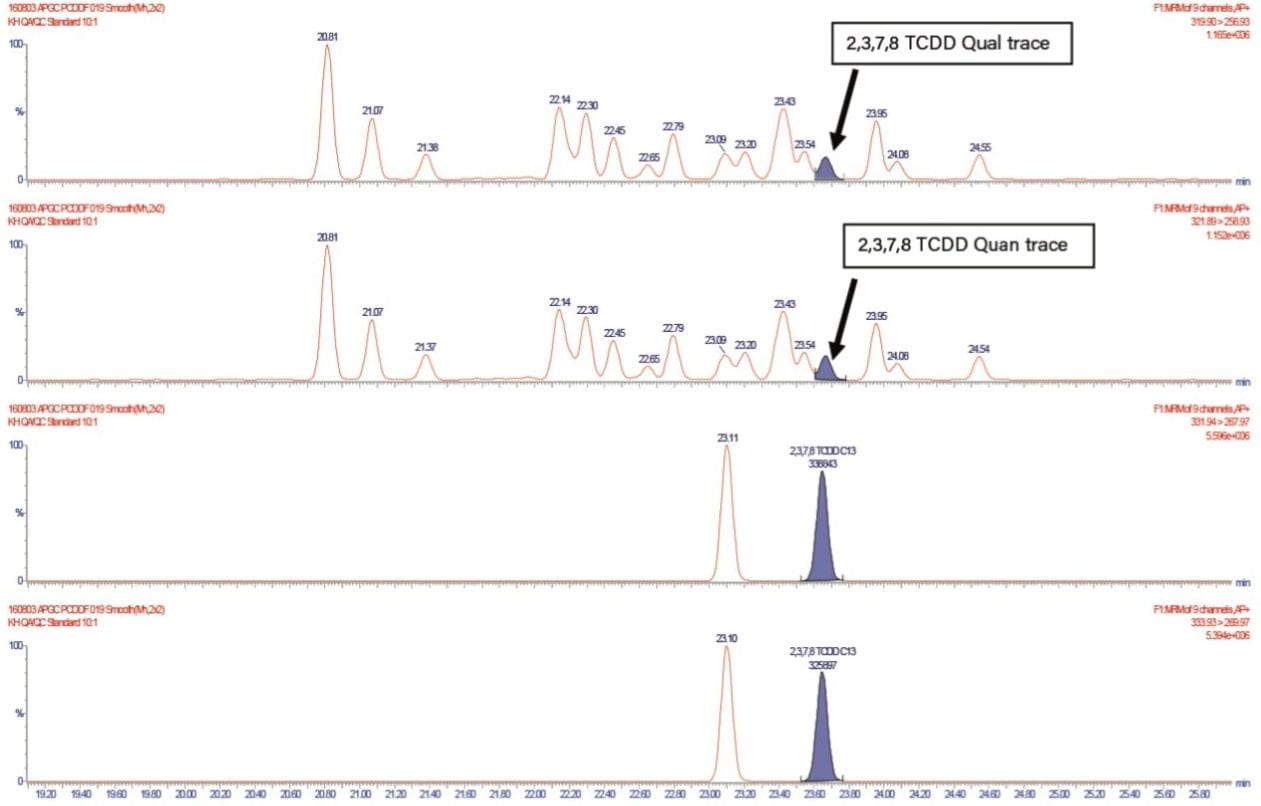
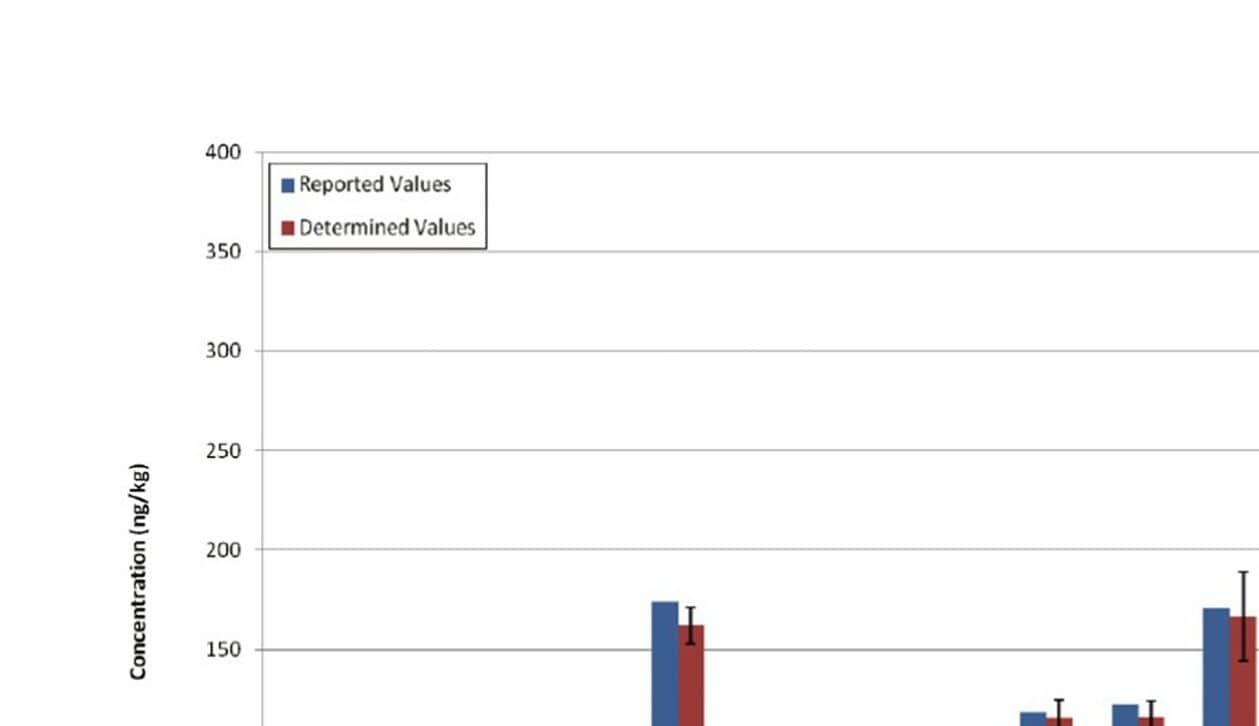
The final assessment was the analysis of a QC fly ash sample. These types of samples are very complex and often used as proficiency tests for dioxin labs in order to ensure that they are producing accurate results. Figure 6 shows the complexity of the samples and demonstrates the ability of APGC and Xevo TQ-XS to quantify the compound of interest, as highlighted in Figure 6. Figure 7 shows that the value obtained with APGC coupled with the Xevo TQ-XS were consistent with that of the QC sample.
Utilizing APGC coupled with the Xevo TQ-XS allows sub-femtogram levels of dioxins to be analyzed in complex samples. The added sensitivity enables the dilution of expensive dioxin standards, reduces the need to pre-concentrate sample extracts (prior to analysis), and minimizes the amount of sample required for testing. Not only is this system exceptionally sensitive, it is also robust and produces consistent results over thousands of injections. The APGC coupled with the Xevo TQ-XS far surpasses the regulatory requirements for dioxin testing.
720006025, August 2017