
The ACQUITY UPLC PLUS Series is specifically designed to enhance the ease of use of UPLC Technology while maintaining the same separation performance of legacy ACQUITY UPLC platforms such as the H-Class and I-Class systems. The objective of this application note is to demonstrate the equivalency and higher robustness of the ACQUITY UPLC PLUS Systems on a variety of biopharmaceutical applications, including glycan analysis, size exclusion chromatography (SEC), and peptide mapping.
With effective treatment for various diseases, biotherapeutics have cultivated a fast growing market requiring instrumentation that is cost-effective to deploy and can deliver robust results in an efficient manner. One way to address the challenges of cost, efficiency, and robustness is the implementation of new technologies throughout the product lifecycle. Liquid chromatography (LC) platforms, as an intrinsic part of the biopharmaceutical development and manufacturing process, are potential candidates for modernization to improve productivity throughout the drug pipeline as part of a pharmaceutical quality system. To this end, UltraPerformance Liquid Chromatography (UPLC) instrumentation has been successfully deployed throughout the development and manufacturing process with substantial gains in assay performance, productivity, and reduced costs. As pharmaceutical companies continue to put more demand on their LC systems to drive products to market faster, instrumentation that offers enhanced robustness and reduced downtime would be beneficial.
The ACQUITY UPLC PLUS Series is specifically designed to enhance the ease of use of UPLC Technology while maintaining the same separation performance of legacy ACQUITY UPLC platforms such as the H-Class and I-Class systems. As shown in Figure 1, multiple enhancements were made on the solvent manager and sample manager of the existing ACQUITY UPLC System portfolio. The change of degasser hardware and firmware can increase the reproducibility of separations and maximize the system uptime, while the new design of the sample manager improves the integrity of thermally-sensitive samples. In addition, the exterior surface treatment of the needle extends its compatibility with additional vial cap and well-plate cover configurations while significantly improving carryover performance. These system enhancements were purposefully engineered to maintain the same system dwell volume and dispersion of the ACQUITY UPLC family members they replace to ensure identical separation profiles could be achieved between platforms. Together, the ACQUITY UPLC PLUS Series enables an enhanced user experience through maximizing uptime with the peace-of-mind to ensure equivalent performance for established methods.

The objective of this application note is to demonstrate the equivalency and higher robustness of the ACQUITY UPLC PLUS Systems on a variety of biopharmaceutical applications, including glycan analysis, size exclusion chromatography (SEC), and peptide mapping. To evaluate the performance of the ACQUITY UPLC PLUS Systems, an ACQUITY UPLC H-Class PLUS Bio System, and an ACQUITY UPLC I-Class PLUS were compared against the ACQUITY UPLC H-Class Bio System and ACQUITY UPLC I-Class System, respectively.
The glycan sample was prepared as previously described.1 Briefly, the RapiFluor-MS Glycan Performance Standard (P/N 186007983) was dissolved in 25 µL of mixed DMF/acetonitrile/water with a ratio of 22.5/55.5/22. LC-MS grade water and acetonitrile was purchased from Fisher Scientific. The 50 mM ammonium formate solvent was prepared by diluting one bottle (10 mL) of Waters Ammonium Formate Solution-Glycan Analysis (P/N 186007081) in 1 L of water.
|
LC system: |
ACQUITY UPLC H-Class Bio ACQUITY UPLC H-Class PLUS Bio |
|
Detectors: |
ACQUITY FLR, 10 mm flow cell, λ = 214 nm |
|
LC column: |
ACQUITY UPLC Glycan BEH Amide, 1.7 μm, 130Å, 2.1 mm × 150 mm (P/N 186004742) |
|
Column temp.: |
60 °C |
|
Sample vial: |
12 x 32 mm glass, Total recovery (P/N 600000750cv) |
|
Injection volume: |
2 μL |
|
Mobile phase A: |
50 mM ammonium formate, pH 4.4 |
|
Mobile phase B: |
Acetonitrile |
|
Time (min) |
Flow rate (min) |
%A |
%B |
|---|---|---|---|
|
Initial |
0.4 |
25 |
75 |
|
35 |
0.4 |
46 |
54 |
|
36.5 |
0.2 |
100 |
0 |
|
39.5 |
0.2 |
100 |
0 |
|
42.5 |
0.2 |
25 |
75 |
|
47.4 |
0.4 |
25 |
75 |
|
55 |
0.4 |
25 |
75 |
As previously described,2 monosodium phosphate, disodium phosphate, and sodium chloride were purchased from Sigma. The mobile phase 20 mM phosphate buffer, was prepared by adding 1.37 g monosodium phosphate (monohydrate), 2.70 g disodium phosphate (heptahydrate), 11.68 g sodium chloride in 900 mL water and adjusting pH to 6.8. The buffer was then diluted to a total volume of 1 L and used as mobile phase. The BEH200 SEC Protein Standard Mix (P/N: 186006518) was fully dissolved in 500 μL mobile phase and used for analysis.
|
LC system: |
ACQUITY UPLC H-Class Bio ACQUITY UPLC H-Class PLUS Bio |
|
Detectors: |
ACQUITY TUV, 10 mm flow cell, λ = 214 nm |
|
LC column: |
ACQUITY UPLC Protein BEH SEC, 200Å, 1.7 μm, 4.6 × 150 mm (P/N 186005225) |
|
Column temp.: |
Ambient |
|
Sample vial: |
12 x 32 mm glass, Total recovery (P/N 600000750cv) |
|
Injection volume: |
4 μL |
|
Mobile phase: |
20 mM phosphate, 200 mM NaCl, pH 6.8 |
|
Flow rate: |
0.885 mL/min |
Trifluoroacetic acid (TFA), LC-MS grade water, and acetonitrile were purchased from Fisher Scientific. Waters MassPREP Enolase Digestion Standard (P/N 186002325) was reconstituted in 100 μL mobile phase A (0.1% TFA in water) and used as sample.
|
LC system: |
ACQUITY UPLC I-Class ACQUITY UPLC I-Class PLUS |
|
Detectors: |
ACQUITY TUV, 10 mm flow cell, λ = 214 nm |
|
LC column: |
ACQUITY UPLC BEH C18, 1.7 μm, 300Å, 2.1 mm × 100 mm (P/N 186002350) |
|
Column temp.: |
65 °C |
|
Sample vial: |
12 x 32 mm glass, Total recovery (P/N 600000750cv) |
|
Injection volume: |
10 μL |
|
Mobile phase A: |
H2O, 0.1% TFA |
|
Mobile phase B: |
Acetonitrile, 0.1% TFA |
|
Time (min) |
Flow rate (min) |
%A |
%B |
|---|---|---|---|
|
Initial |
0.200 |
99.0 |
1.0 |
|
3.00 |
0.200 |
99.0 |
1.0 |
|
88.00 |
0.200 |
50.0 |
50.0 |
|
90.00 |
0.200 |
10.0 |
90.0 |
|
100.00 |
0.200 |
10.0 |
90.0 |
|
102.00 |
0.200 |
99.0 |
1.0 |
|
126.00 |
0.200 |
99.0 |
1.0 |
As a critical quality attribute (CQA), it is essential to characterize and monitor glycoprofiles in biopharmaceutical development for consistency to ensure product safety and efficacy. As part of the glycan profiling workflow, Hydrophilic Interaction Chromatography (HILIC) of released glycans is extensively used and often requires long and shallow gradient elution due to the complexity of the glycoprofiles. To this end, a highly robust LC instrument is essential to perform glycan profiling.
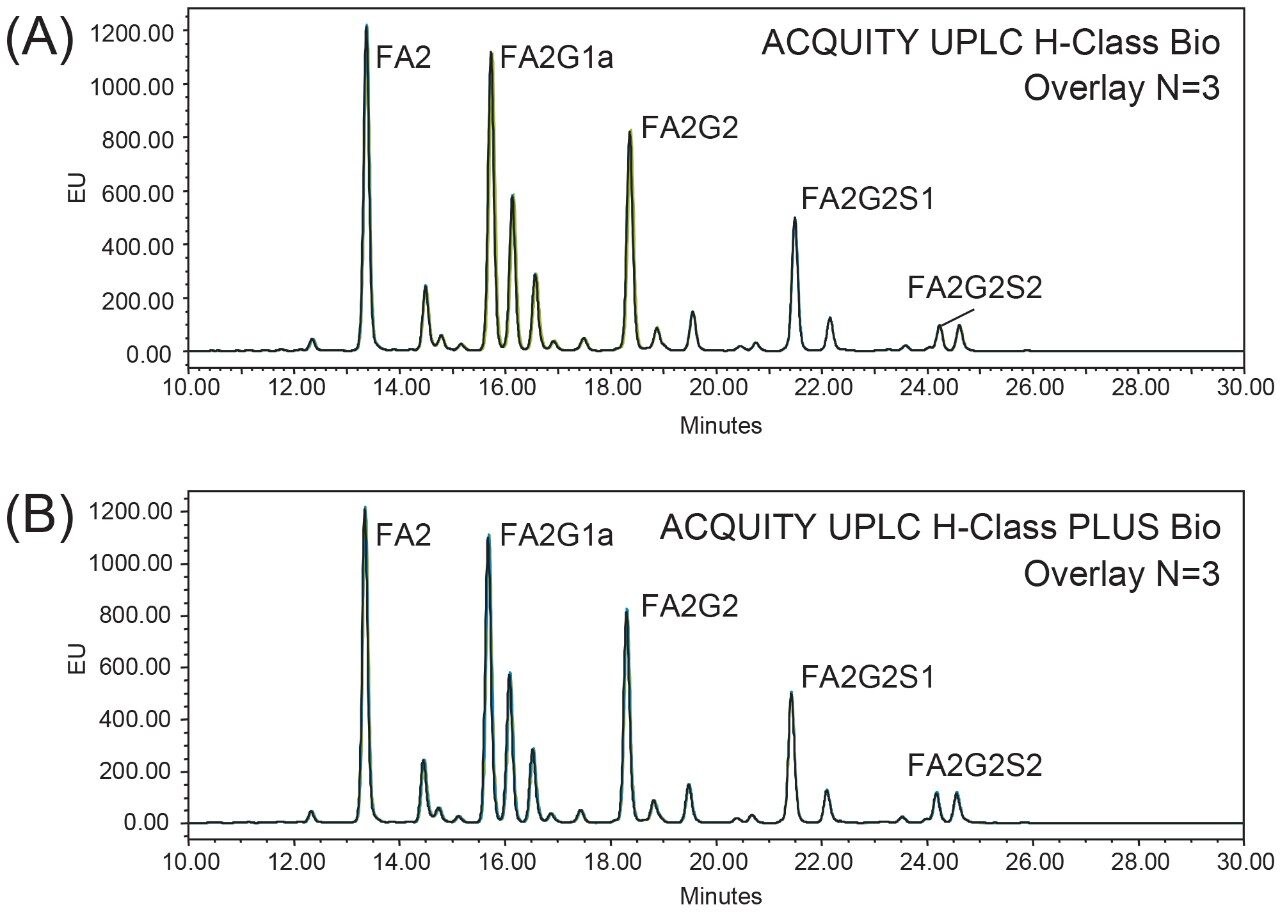
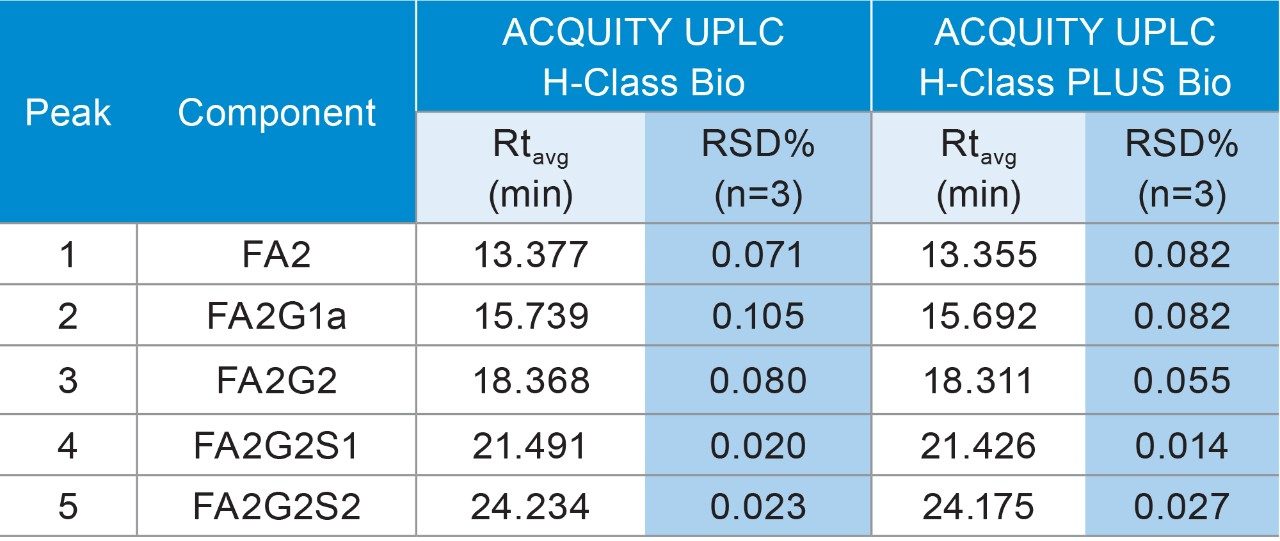
To evaluate the performance of the ACQUITY UPLC PLUS System for glycan analysis, the RapiFluor-MS Glycan Performance Test Standard was separated by a 15 cm ACQUITY UPLC Glycan BEH Amide Column on a conventional ACQUITY UPLC H-Class Bio System to establish a baseline.1 Using a gradient from 46 to 25% acetonitrile in 35 min, all glycans were well separated in a 20 min elution window, as shown in Figure 2A. The same experiments were then performed on an ACQUITY UPLC H-Class PLUS Bio System for comparison. As shown in Figure 2B, the same separation profile was obtained, while the retention times for the five most abundant peaks recorded in Table 1 were highly comparable with previous results, demonstrating the equivalency of the two LC systems. Robustness was also evaluated for the two systems by calculating the %RSD of retention times for the five peaks based on three consecutive injections. As listed in Table 1, the %RSD of retention time on the ACQUITY UPLC H-Class PLUS Bio System (no more than 0.082%) was systematically smaller than the conventional ACQUITY UPLC H-Class Bio System (no more than 0.105%), suggesting reproducibility improvement of the upgraded UPLC system.
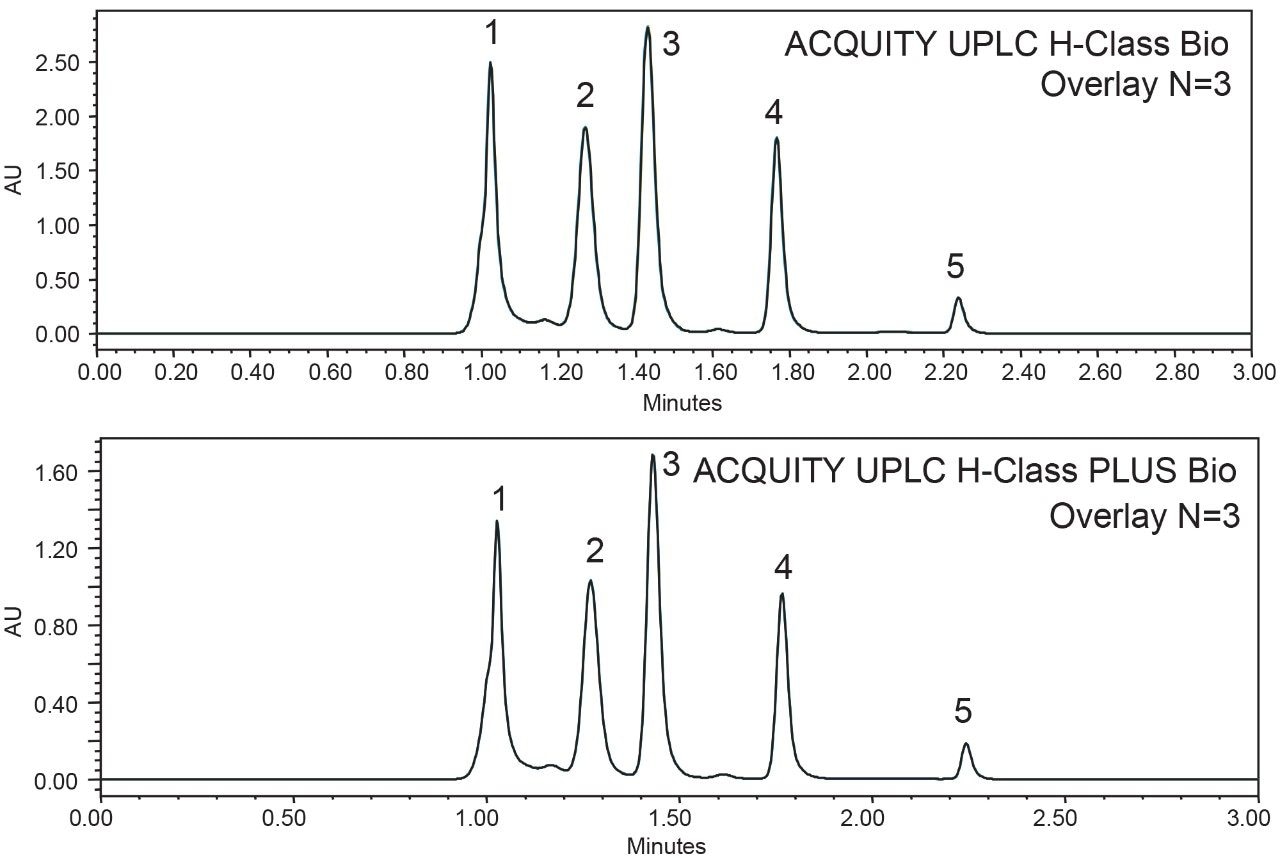
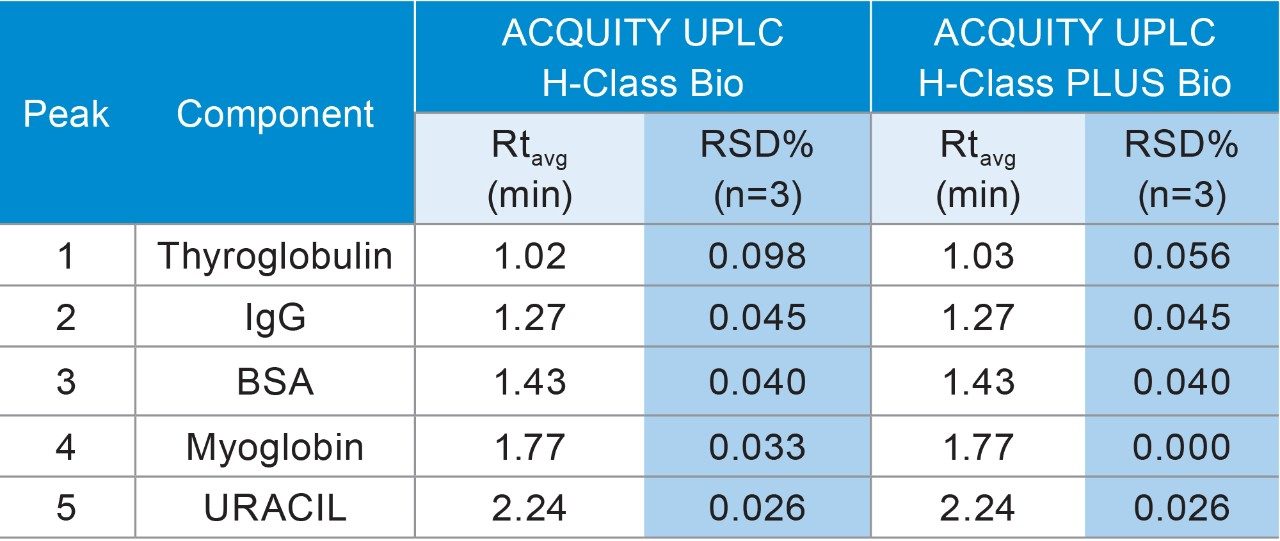
SEC is extensively used in characterization and routine monitoring of biopharmaceuticals. Reproducible retention time in SEC is essential to accurately determine the aggregation and fragmentation of the drug substance. To compare the performance of the ACQUITY UPLC H-Class Bio System and the ACQUITY UPLC H-Class PLUS Bio System, the SEC200 protein mixture standard was used as a test sample and separated on a 15 cm ACQUITY UPLC Protein BEH SEC column.2 As shown in the overlaid chromatograms (n=3) in Figure 3, the five components were well resolved in three minutes with nearly identical retention times obtained on both instruments for each component (Table 2), suggesting equivalency of the two systems. Table 2 also listed calculated %RSD of the retention time for the five peaks, which demonstrated the higher reproducibility of the ACQUITY UPLC H-Class PLUS Bio System for SEC applications.
In biopharmaceutical development, enzymatic digestion of proteins followed by peptide mapping is a well established approach for characterization and monitoring of product CQAs. Depending on enzyme cleaving sites and size of the protein, the enzymatic digests can be highly complex to resolve. In this case, the chance of co-elution of closely eluted peaks becomes more of a concern, requiring reproducible retention time for accurate peak identification and integration.
To obtain the most reproducible results, peptide mapping of the Waters MassPREP Enolase Digestion Standard was performed on an ACQUITY UPLC I-Class System for its design of binary pump and high pressure mixing. To ensure the maximum amount of coverage, a 75 min gradient starting from 1% to 50% acetonitrile was used to achieve high resolution and retain the polar peptides.3 As demonstrated in Figure 4, highly comparable peptide mapping profiles were obtained, indicating the suitability of replacing the conventional ACQUITY UPLC I-Class System with the ACQUITY UPLC I-Class PLUS System. To further compare the instrument performance, five peaks were selected to measure the reproducibility across the gradient. As shown in Table 3, the %RSD of retention time was significantly reduced on the ACQUITY UPLC I-Class PLUS System (maximum %RSD from 0.044% to 0.014%) while the average retention times remained comparable. Together, these data demonstrated the consistency of separation performance and improvements of reproducibility using the ACQUITY UPLC I-Class PLUS System.
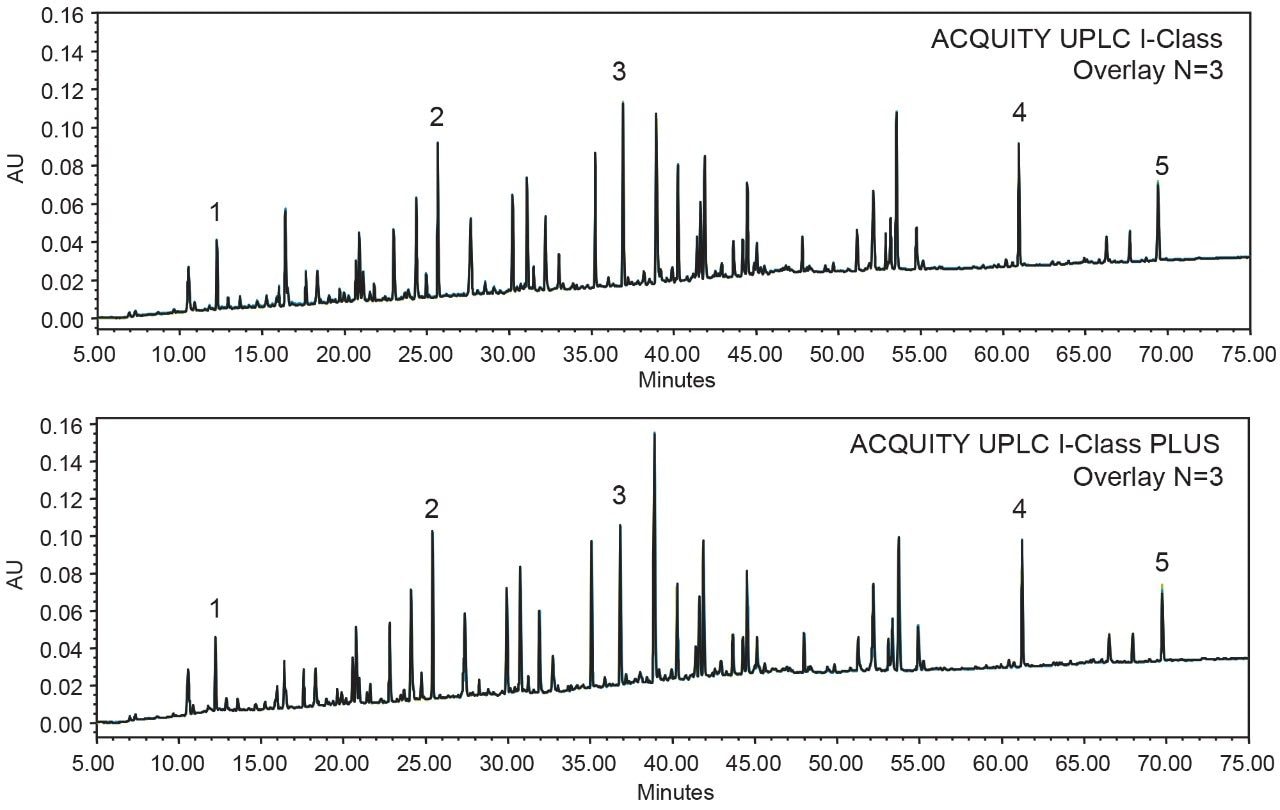
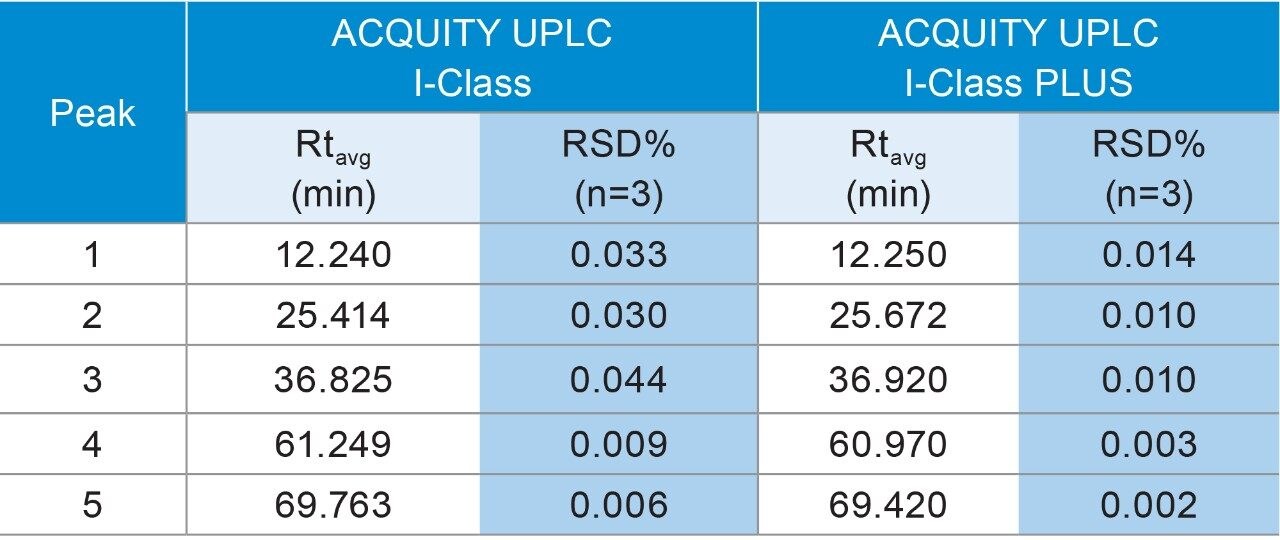
In this work, the comparability and robustness of the ACQUITY UPLC and the ACQUITY UPLC PLUS systems were investigated using techniques frequently employed in the development and manufacturing of biopharmaceutical products. While maintaining the same separation profile, ACQUITY UPLC H-Class PLUS Bio System reduced the %RSD for both glycan analysis and SEC. Improvement of robustness was also found on ACQUITY UPLC I-Class PLUS System with %RSD of average retention times below 0.014% for peptide mapping. To summarize, the ACQUITY UPLC PLUS systems provide equivalent separation performance with higher reproducibility compared to conventional UPLC systems and can be used to improve the robustness of biopharmaceutical applications.
720006285, May 2018