
A UPLC method was successfully developed for the analysis of active pharmaceutical ingredients in common over-the-counter cold and flu drug formulations. In this application note, we present the development of a MS-compatible UPLC method for the simultaneous determination of eight active pharmaceutical ingredients (APIs) found in common over-the-counter (OTC) cold and flu medication. These APIs include acetaminophen, dextromethorphan hydrobromide, phenylephrine hydrochloride, chlorpheniramine maleate, ibuprofen, pseudoephedrine hydrochloride, duaifenesin, and doxylamine succinate.
Pharmaceutical drug products used for treatment of common cold and flu often contain multiple active ingredients to target different symptoms and may include combination of decongestants, antihistamines, pain relievers, cough suppressants, and expectorants. These actives often exhibit different chemical characteristics such as a wide range of polarities, making chromatographic method development a challenging task.
Many of the methods reported in the literature for cold and flu medication are designed for analysis of individual components or for a particular dosage form. While other methods are suitable for simultaneous determination of multi-components in drug formulations, they utilize mobile phases with non-volatile buffers that are not compatible with mass detection.1-3 Addition of mass detection to the pharmaceutical analysis workflows can often enable a quick and accurate identification of new or unknown sample components during the development or to confirm peak purity in routine assay testing.
In this application note, we present the development of a MS-compatible UPLC method for the simultaneous determination of eight active pharmaceutical ingredients (APIs) found in common over-the-counter (OTC) cold and flu medication. These APIs include acetaminophen, dextromethorphan hydrobromide, phenylephrine hydrochloride, chlorpheniramine maleate, ibuprofen, pseudoephedrine hydrochloride, duaifenesin, and doxylamine succinate. A systematic protocol that includes scouting, screening, and optimization steps is employed to ensure faster and more effective development of robust and reproducible method. Results from each step are analyzed using custom calculations and custom reports of the Empower 3 Chromatographic Data Software. Both the UV and mass spectral data from the ACQUITY QDa Mass Detector is utilized for accurate identification and tracking all of the components during the development process. Finally, the developed method is used to analyze commercially available over-the-counter cold and flu medication.
Separate stock solutions were prepared in methanol at 1.0 mg/mL. An equal volume of each stock solution was transferred to one vial and diluted with a standard diluent (90:10 water/methanol) to a final working concentration of 100 µg/mL of each analyte.
Over-the-counter cold and flu medication tested in this study included syrup, tablets, and caplets. All samples were prepared and diluted to the working concentration with sample diluent containing 90:10 water/methanol as outlined in Table 2. Each working solution was then filtered through 0.2 µm GHP syringe filter prior to analysis.
|
LC system: |
ACQUITY UPLC H-Class PLUS with Column Manager (Active) and Solvent Select Valve |
|
Columns: |
All columns with dimension of 2.1 × 50 mm |
|
|
ACQUITY UPLC CSH C18, 1.7 μm |
|
|
ACQUITY UPLC CORTECS™ T3, 1.6 μm |
|
|
ACQUITY UPLC CORTECS Phenyl, 1.6 μm |
|
|
ACQUITY UPLC HSS PFP, 1.8 μm |
|
|
ACQUITY UPLC BEH C18, 1.7 μm |
|
|
ACQUITY UPLC BEH Shield RP18, 1.7 μm |
|
Column temp.: |
40 °C |
|
Injection volume: |
1.0 μL |
|
Flow rate: |
0.6 mL/min |
|
Mobile phase A: |
125 mM Formic acid in water |
|
Mobile phase B: |
125 mM Ammonium hydroxide in water |
|
Mobile phase C: |
Water |
|
Mobile phase D1: |
Acetonitrile |
|
Mobile phase D2: |
Methanol |
|
Separation: |
Gradient with 5–90% organic solvent over 5 minutes |
|
Purge/sample wash solvent: |
70:30 water/methanol |
|
Seal wash: |
90:10 water/acetonitrile |
|
UV detector: |
ACQUITY UPLC PDA 200–500 nm (Derived at 220 nm) |
|
Column: |
ACQUITY UPLC BEH C18, 2.1 × 50 mm, 1.7 um |
|
Column temp.: |
40 °C |
|
Injection volume: |
1.0 μL |
|
Flow rate: |
0.6 mL/min |
|
Mobile phase A: |
10 mM Ammonium acetate in water with 0.2% of ammonium hydroxide |
|
Mobile phase B: |
Methanol with 0.2% ammonium hydroxide |
|
Purge/sample wash solvent: |
70:30 water/methanol |
|
Seal wash: |
90:10 water/acetonitrile |
|
PDA settings: |
200–500 nm (derived at 215 nm) |
|
Step |
Time (min) |
Solvent A (%) |
Solvent B (%) |
|---|---|---|---|
|
1 |
Initial |
95.0 |
5.0 |
|
2 |
5.0 |
10.0 |
90.0 |
|
3 |
5.5 |
10.0 |
90.0 |
|
4 |
5.6 |
95.0 |
5.0 |
|
5 |
7.5 |
95.0 |
5.0 |
|
MS detector: |
ACQUITY QDa (Extended Performance) |
|
Ionization mode: |
ESI+, ESI- |
|
Acquisition range: |
100–400 m/z |
|
Capillary voltage: |
0.8 kV (pos/neg) |
|
Cone voltage: |
5 V |
|
Probe temp.: |
600 °C |
|
Data: |
Centroid |
|
System control, data acquisition, and analysis: |
Empower 3 FR4 Chromatography Data Software (CSD) |
A UPLC method for analysis of APIs found in common over-the-counter (OTC) cold and flu medication was developed following a systematic protocol.4 A systematic protocol is based on a consistent evaluation of major selectivity factors, which enables development of robust and reliable methods.
Before beginning the study, we defined the separation goals and selected a chromatographic system. The goal of our study was to separate all APIs with minimum resolution of ≥2.0 between the peaks, peak tailing of ≤1.5, and retention factor (k*) ≥2.0. We used the ACQUITY UPLC H-Class PLUS System configured with a Column Manager and Solvent Select Valve to maximize flexibility of the method development. The ACQUITY QDa Mass Detector in combination with the ACQUITY PDA Detector allowed quick identification of all components and possible co-elution during the development process.
After defining the separation goals and system, we started the study following a three step approach that included rapid scouting, screening, and optimization.
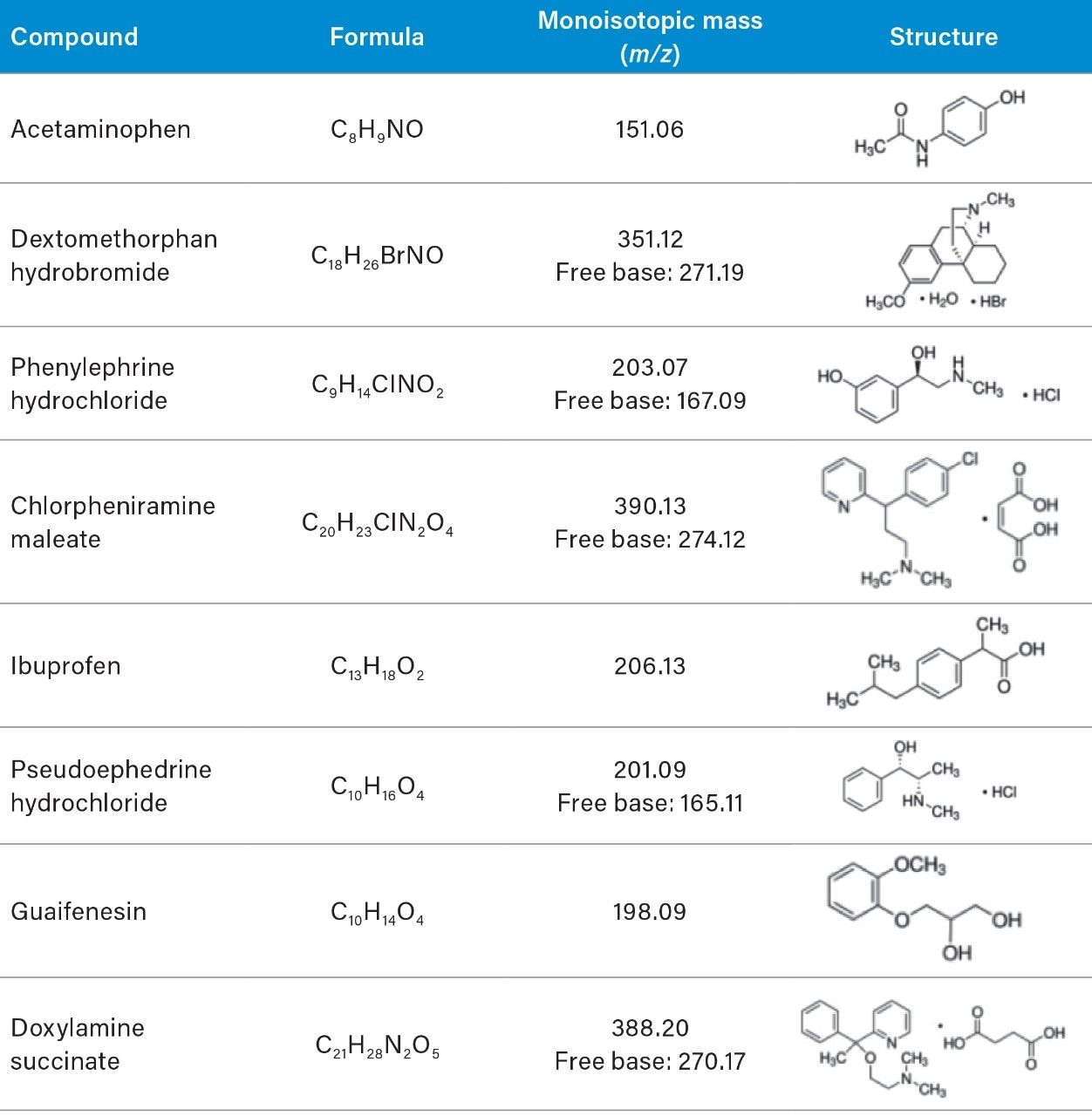
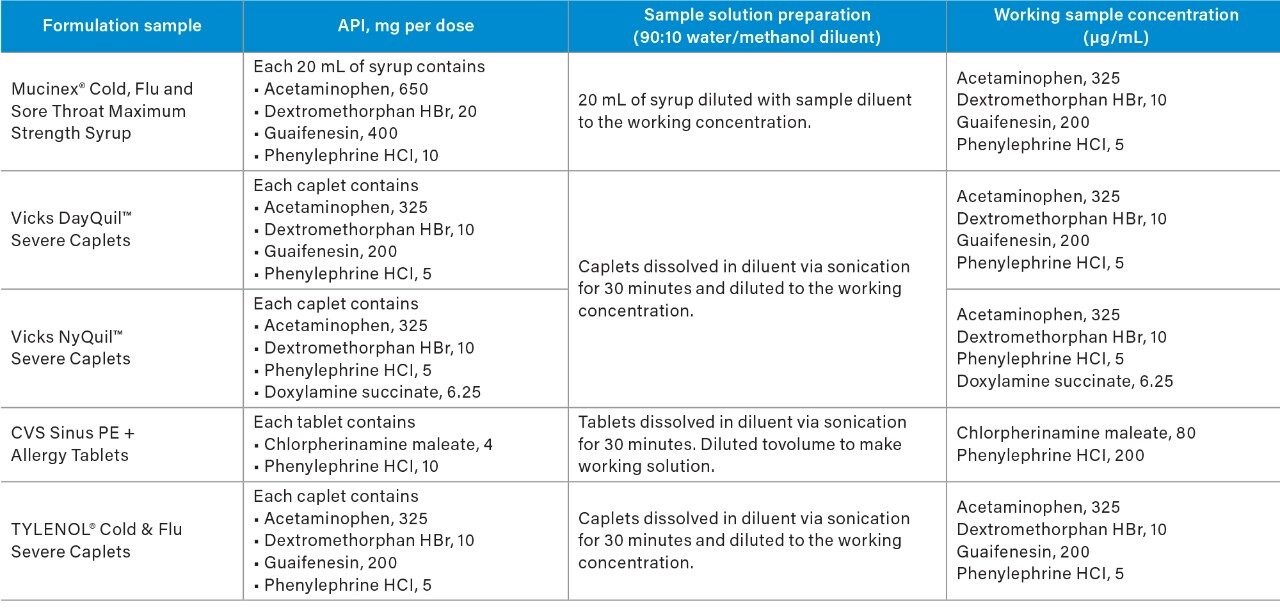
The goal of the rapid scouting was to quickly identify separation condition that provided the best retention of our analytes, as well as to determine the best separation mode (reverse-phase or HILIC). The low and high pH experiments were performed on an ACQUITY UPLC CSH C18 Column using stock solutions of 125 mM formic acid and 125 mM ammonium hydroxide with a gradient of 5–90% of acetonitrile solvent over five minutes. The chromatographic data showed that the retention of our analytes changed under low and high pH conditions (Figure 1). The mass data of the ACQUITY QDa was used to identify each peak by mass-to-charge (m/z) ratio (Figure 1A). The MS Peak Tracking feature and report of the Empower 3 Software enabled us to monitor elution order of each analyte over the pH experiments (Figure 1B). The report table displayed the retention time for each peak with a specific m/z over the chromatographic runs.
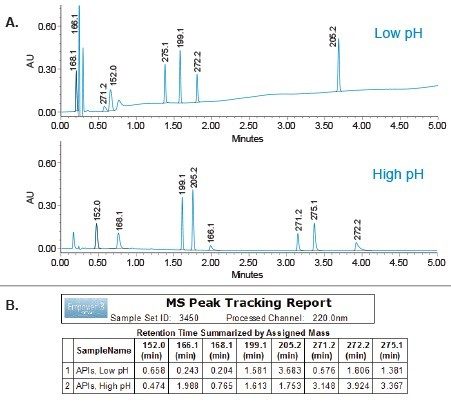
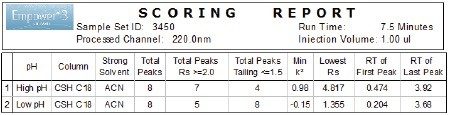
Custom calculations and custom scoring report of the Empower 3 Software were used to quickly identify which condition provided best separation (Figure 2). The low and high pH separations were scored for best conditions by identifying number of peaks that met the performance goals. In this case, the high pH provided best retentivity for all analytes, hence was chosen for the next step of the method development.
The high pH condition from the scouting step was screened with an ACQUITY UPLC CSH C18 and ACQUITY UPLC BEH C18 columns using methanol and acetonitrile solvents, respectively (Figure 3). The separation was performed using the same gradient as in the rapid scouting step. The chromatographic data showed that each condition provided an acceptable separation between components (Figure 3A). The scoring report was used to analyze the chromatographic data and showed that the ACQUITY UPLC BEH C18 with methanol provided best separation with highest number of peaks with the USP resolution ≥2.0 and a tailing ≤1.5 (Figure 3B). Using this condition, we moved forward to the optimization phase.
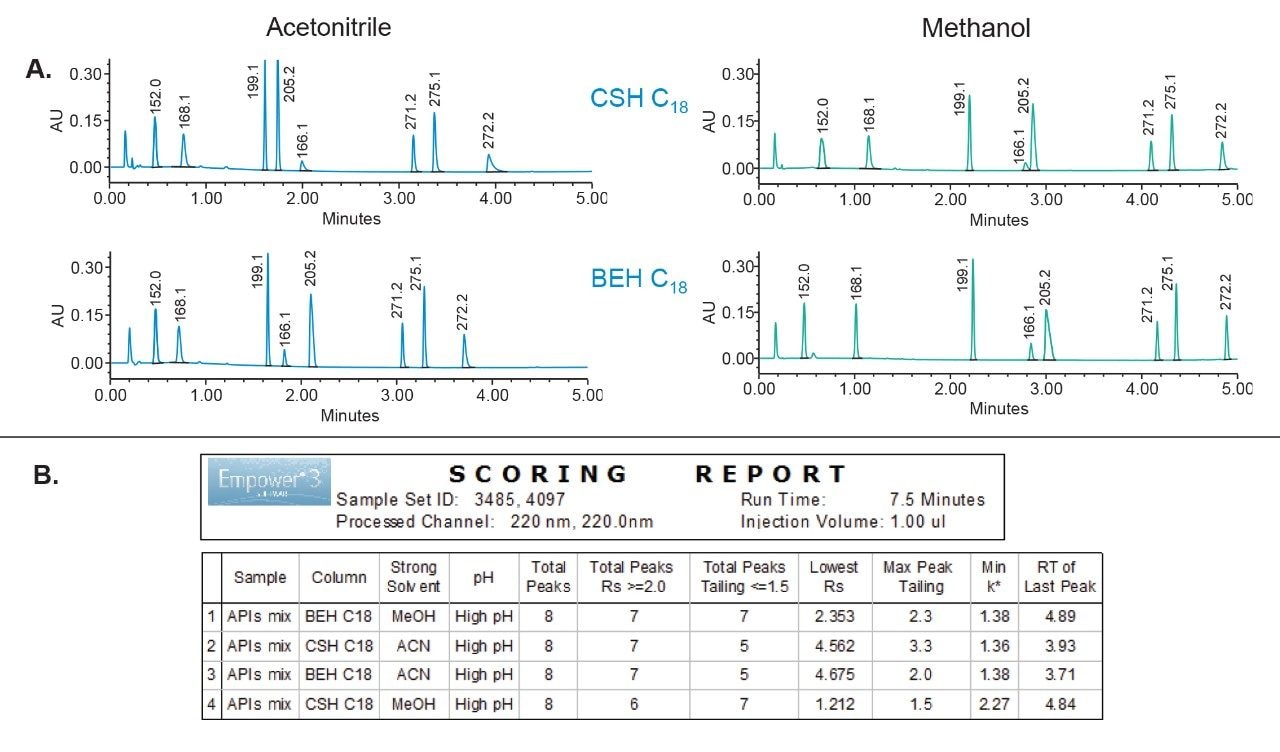
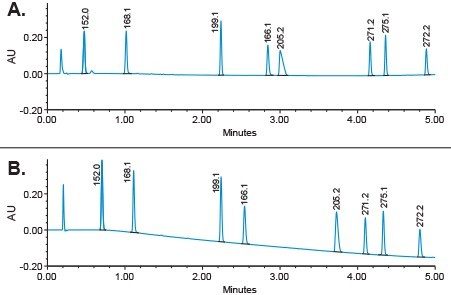
Next, we optimized different chromatographic parameters including gradient slope, column temperature, pH, and wavelength. In addition, we investigated addition of MS-compatible buffers to the mobile phase to further improve separation and peak tailing for our analytes. It was found that addition of ammonium acetate to the mobile phase with 0.2% ammonium hydroxide improved chromatographic separation and reduced peak tailing (Figure 4).
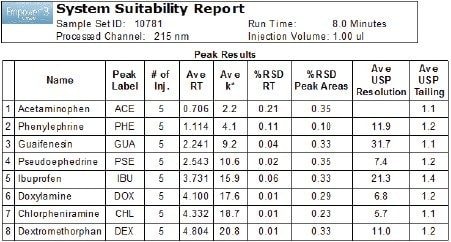
Performance of the developed UPLC method was measured by evaluating repeatability of five replicate injections of the sample according to the specifications defined in the USP General Chapter <621> Chromatography.5 The system suitability results (Figure 5) showed excellent repeatability of the retention times and peak areas with peak tailing ≤1.4.
In addition, reproducibility of the final method was investigated across three column batches using the ACQUITY UPLC BEH C18 method validation kit (MVK). The ACQUITY UPLC method validation kits provide three batches of chromatographic media to judge the quality, reliability, and consistency of an analytical method. Reproducibility of the chromatographic separation across the three batches of the ACQUITY UPLC BEH C18 Column was excellent (Figure 6).

The over-the-counter cold and flu samples were analyzed to show that the develop method can separate active components from the formulation excipients. This is consistent with method specificity and often done by verifying peak purity or spectral homogeneity of the chromatographic peak.
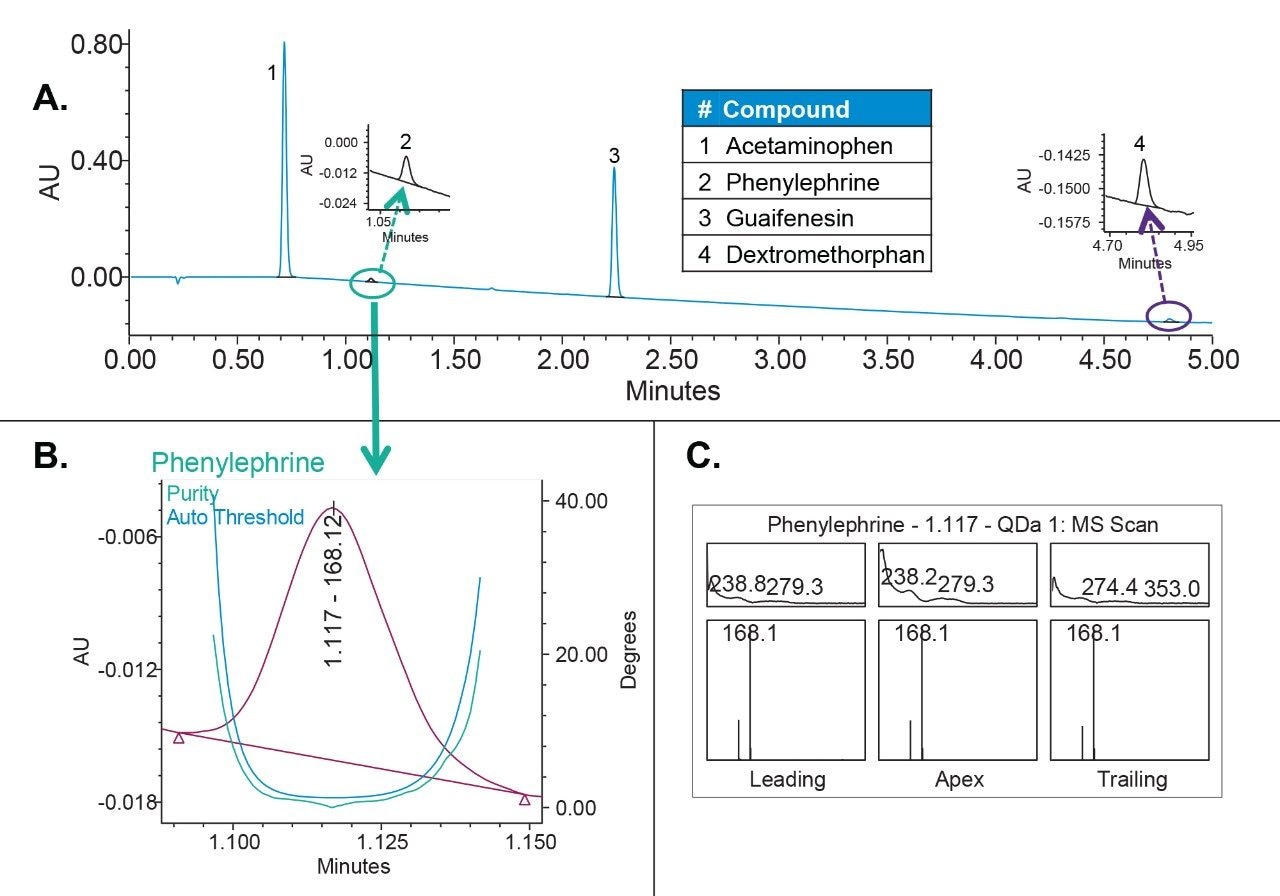
In our study, we used UV in combination with MS spectral data to demonstrate that active components in cold and flu formulations were spectrally homogenous. As for the example, we are showing peak homogeneity determination of phenylephrine peak in Mucinex syrup sample (Figure 7). The UV peak purity plot showed that the peak purity angle was below the threshold angle, indicating that the phenylephrine was spectrally homogenous (Figure 7B). The Empower 3 Mass Analysis Window enabled us to look at the peak purity spectrum across each peak; that is at the leading, apex and trailing edge of the peak (Figure 7C). The top plot represents UV spectrum and the bottom MS spectrum, respectively. The MS spectrum showed presence of one mass (m/z) across the entire peak, specific for phenylephrine. Overall, both the UV peak purity plot and mass spectral data confirmed that the phenylephrine was not coeluting with the components of the sample formulation.
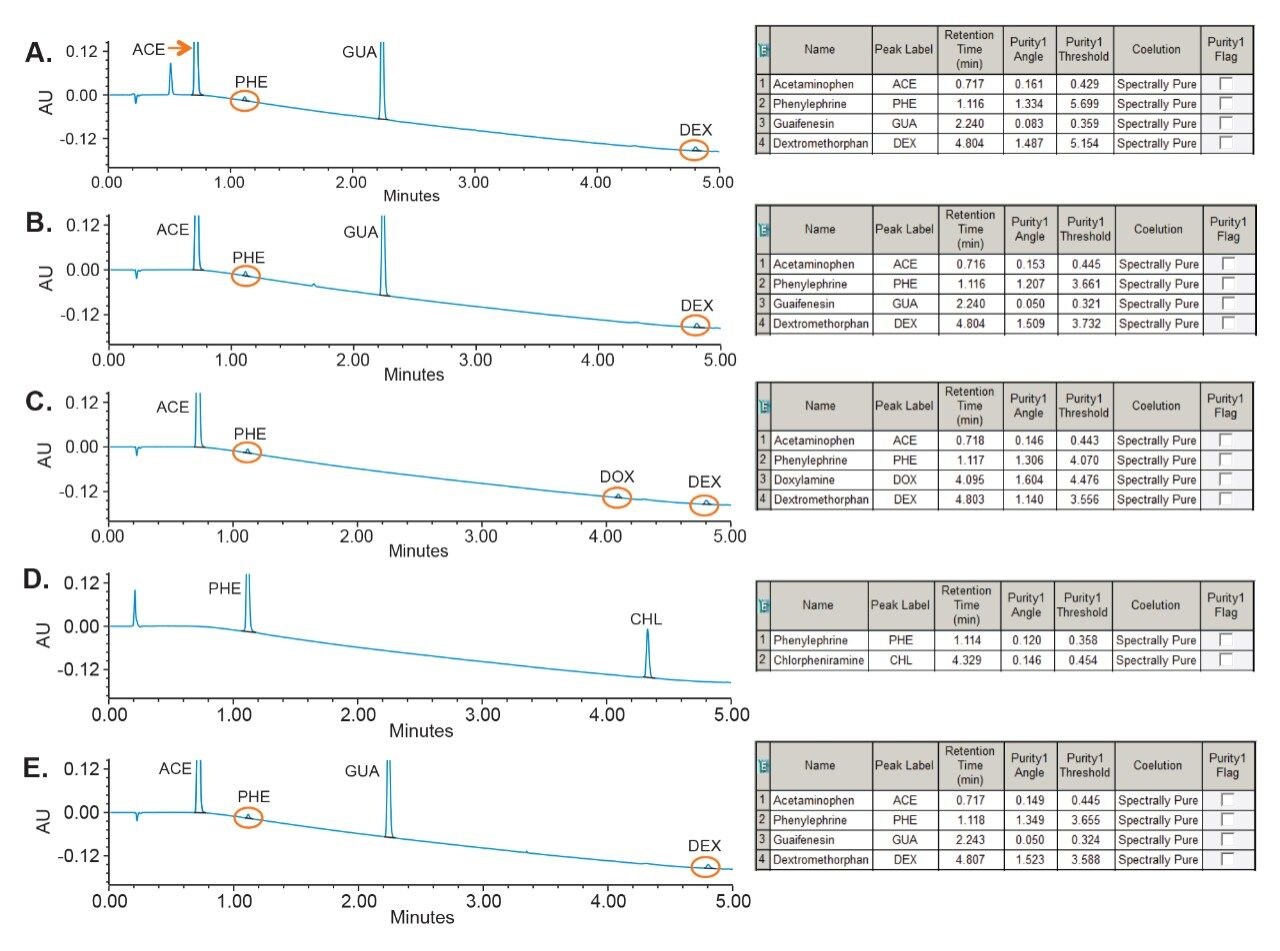
Overall, the analysis of all cold and flu drug commercially available medication showed that the active components were not subject to the interference with excipients of syrup, tablets, and caplets formulations (Figure 8). The Empower peak table for each medication showed that the purity angle was below the threshold angle, confirming that each active is spectrally homogenous or not coeluting with other components of the sample formulation.
A UPLC method was successfully developed for the analysis of active pharmaceutical ingredients in common over-the-counter cold and flu drug formulations.
The ACQUITY UPLC H-Class PLUS with Column Manager and Solvent Select Valve streamlined method development by allowing us to screen multiple columns with different mobile phase in one chromatographic run. The ACQUITY QDa in conjunction with UV detection enabled quick identification of sample components and monitoring elution order of peaks during the study. This eliminated the need to run multiple injections to identify peaks by retentions times.
The MS peak tracking in Empower Software simplified method development by accurately tracking retention times of each component in sample by mass detection. Empower custom calculations and custom scoring report facilitated quick selection of best conditions at each step of the method development. The Empower Software peak purity determination confirmed spectral homogeneity of each active component in the pharmaceutical formulations.
Overall, combining ACQUITY UPLC PLUS System with UV and mass detection enables analytical laboratories to quickly and efficiently develop chromatographic methods.
720006523, March 2019