This application brief demonstrates the use of Empower 3 Software to confirm spectral purity of a chromatographic peak using PDA and mass spectral data.
Empower Chromatography Data Software enables users to verify spectral purity of a chromatographic peak using PDA and mass spectral data.
Specificity of analytical methods used for quality testing of drug products must be demonstrated to assure that the desired analytes are separated and not subject to interference with other components of the sample matrix. When peaks go undetected or potential co-elution unidentified, it may compromise safety and efficacy of the pharmaceutical products. Therefore, confirming spectral purity or homogeneity will help to identify whether a chromatographic peak represents of a single compound or is coeluting with other species of the sample. In addition, performing a peak purity check prior to analytical quantitation helps to assure accuracy of the reportable results.
This technology brief illustrates use of the peak purity tools in the Empower 3 Software to verify spectral purity of the active pharmaceutical ingredients (APIs) within a Mucinex syrup sample.¹ Using both the UV spectral data from an ACQUITY PDA Detector and mass spectral data from an ACQUITY QDa Mass Detector provides confirmation that each active ingredient is not coeluting with any other components of the syrup sample.
The peak purity tools in the Empower Software determines whether a chromatographic peak is comprised of a single component or is spectrally homogenous by comparing spectra from each data point across the entire peak against the reference spectrum at the apex. When the UV spectra at all points across the peak are the same, the chromatographic peak represents a single compound. However, changes of UV spectra across the peak indicate that the peak is comprised of more than one component or is subject to coelution with other species in the sample injection.²
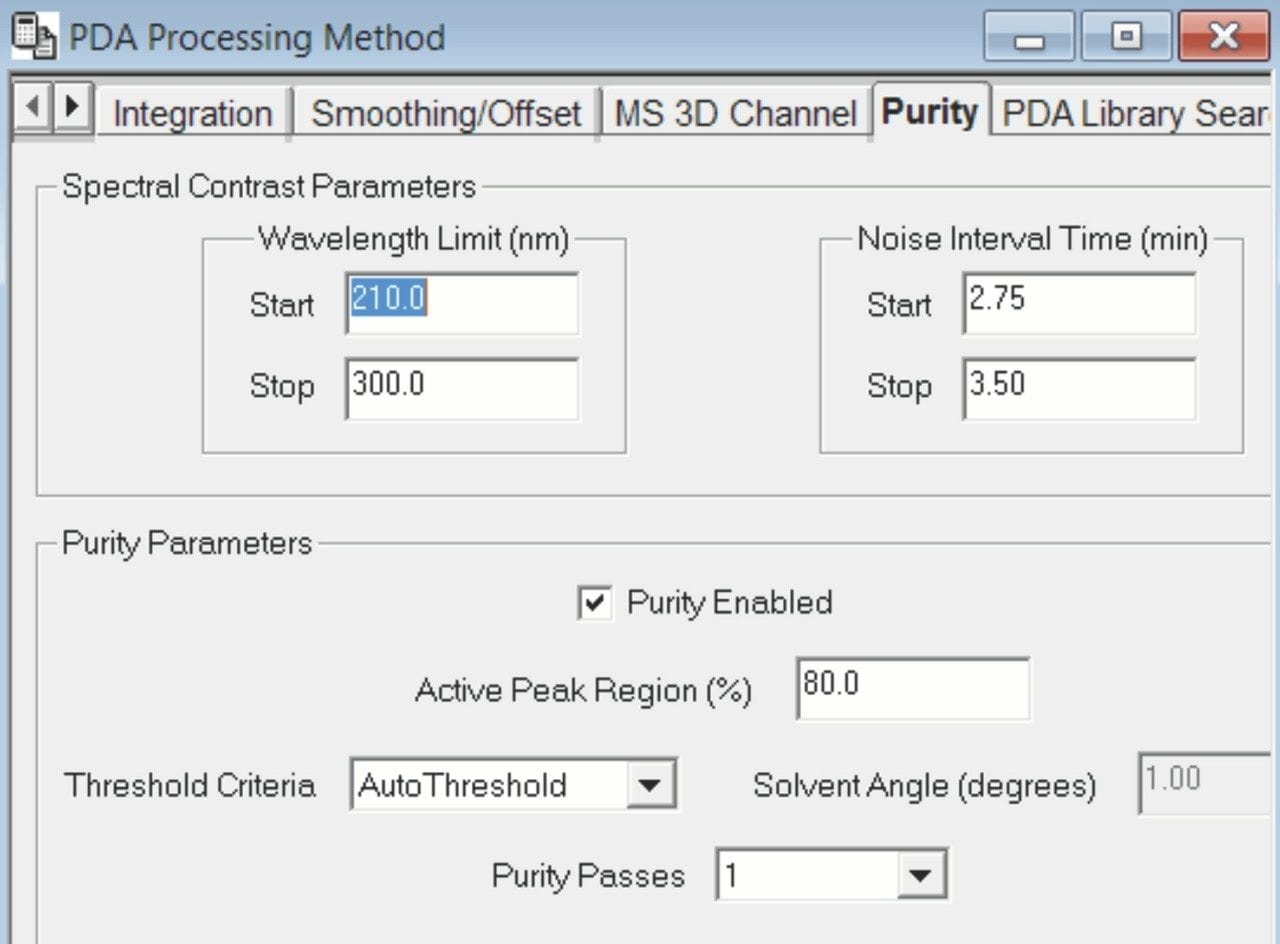
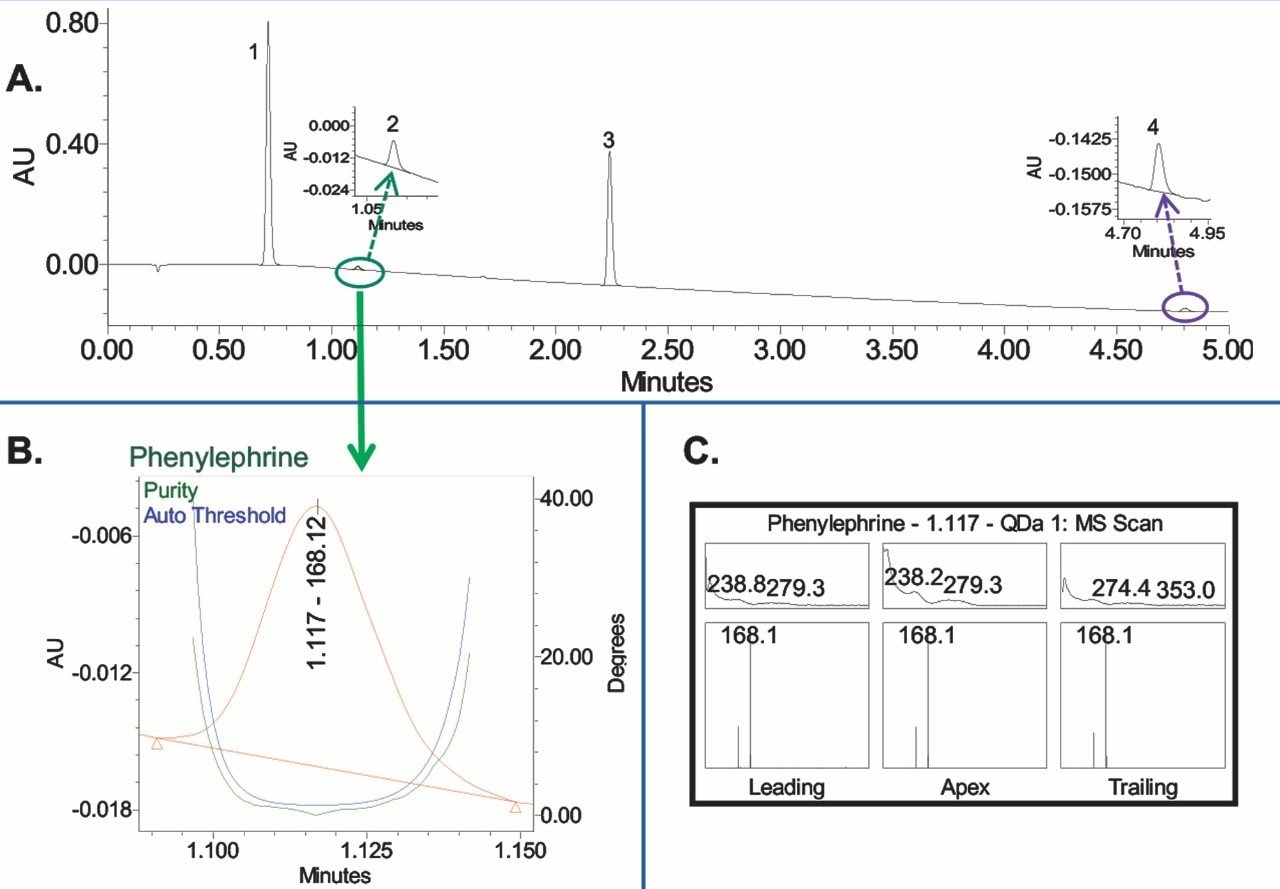
In this study, we verify spectral purity of the APIs within Mucinex syrup formulation using both the PDA and MS spectral data.¹ First, we enable the purity function in the Empower processing method and define the wavelength and noise values (Figure 1). The peak purity of the APIs in the syrup sample is then evaluated (Figure 2). For example, the UV purity plot shows that the phenylephrine peak purity angle is below the threshold angle, indicating that the phenylephrine is spectrally homogenous and not subject to coelution with any other components in the sample (Figure 2B). The peak purity spectra of the Empower 3 Mass Analysis Window shows the PDA and MS spectral data in one plot across the entire peak, which is at the leading, apex, and trailing edge of the chromatographic peak (Figure 2C). The MS spectra shows presence of one mass (m/z) across the entire peak, specific for phenylephrine. Both the UV peak purity plot and mass spectral data confirmed that the phenylephrine is spectrally homogenous and not coeluting with the other components of the syrup formulation.
Overall, the analysis of the syrup sample shows that the purity angle of each API is below the threshold angle, demonstrating spectral homogeneity (Figure 3).
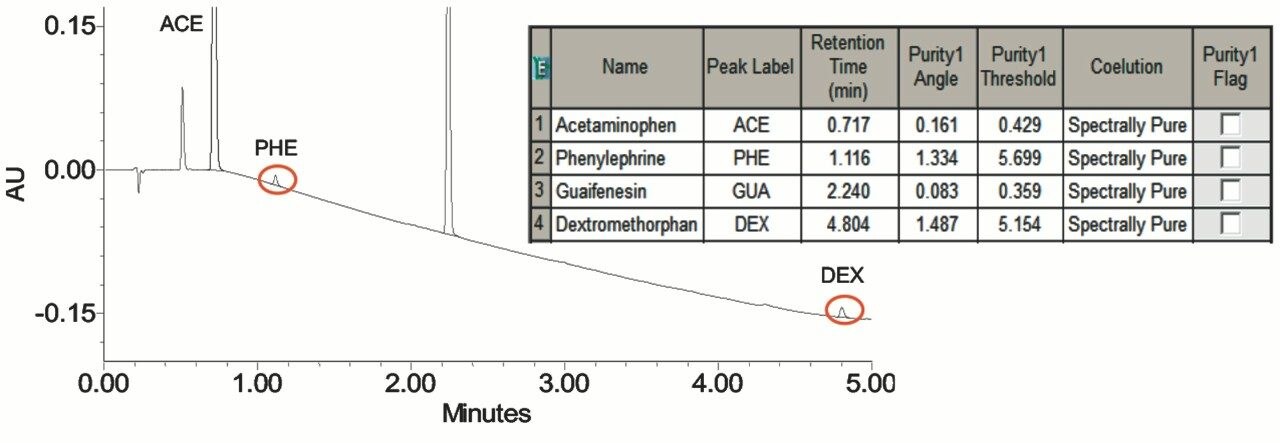
The Empower 3 Software peak purity determination verified spectral homogeneity of the active pharmaceutical ingredients within the Mucinex syrup sample. This confirmed that the active compounds are successfully separated and not coeluting with other components in the formulation.
Utilizing both UV and MS spectral data increases confidence and assurance in determination of the chromatographic peak purity, especially for species with comparable structures. Accurate assessment of spectral peak purity is critical to the assurance of the safety and efficacy of the drug products.
720006582, May 2019