This is an Application Brief and does not contain a detailed Experimental section.
Analytical methods used for testing of pharmaceutical raw materials and finished drug products are often transferred across organizations or to contract partners that utilize instruments from different vendors. In addition, when a new modern instrument is introduced into a laboratory, the quality department needs confidence that the new instrument can accurately run existing methods. It is essential that these methods be successfully transferred between different instruments to ensure product consistency and regulatory compliance. This application brief demonstrates seamless transfer of an HPLC impurities method to an Arc HPLC System and to a smaller particle column for improved laboratory efficiency and productivity.
Effective method transfer produces equivalent results for the same analysis, independent of the instrument, laboratory, or the resources. This is important to minimize the need to revalidate the method, which can be time consuming and costly. In this work, we present seamless transfer of an HPLC method for impurities analysis to a Waters Arc HPLC System. Furthermore, we show that scaling the method from 5 to 3.5 µm and keeping the same flow rate reduces run time and solvent consumption, without compromising the integrity of the chromatographic separation. The success of the method transfer is assessed by examining chromatographic separation and the system suitability results according to the USP recommendations.1
The Arc HPLC System seamlessly replicates HPLC methods without compromising the chromatographic separation quality or requiring any revalidation effort. It also accommodates the use of 3.5 µm particle size columns, with appropriate scaling and maintaining the same flow rate, to increase laboratory efficiency and productivity.
In addition, the higher back pressure tolerance of the Arc HPLC allows methods to leverage increased resolving power of 3.5 µm particle size columns, without changing column length, flow rate, or gradient conditions.
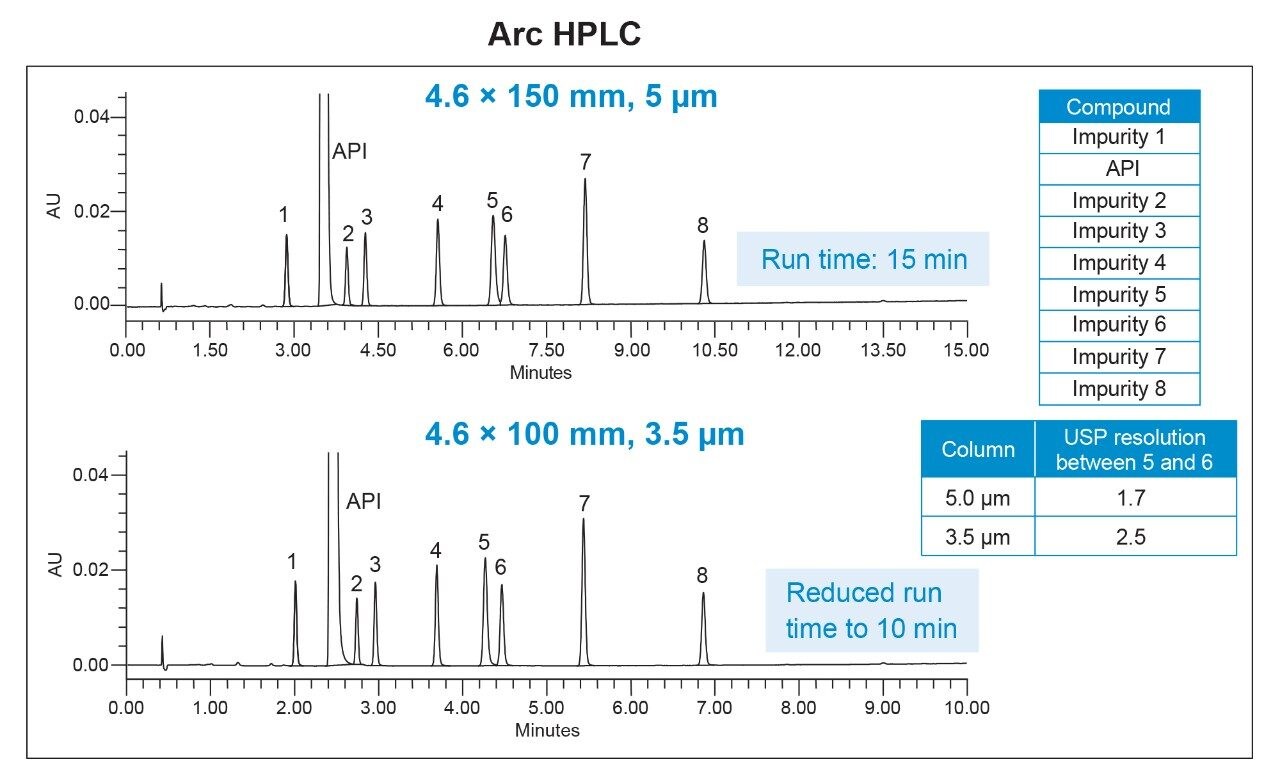
The HPLC method for impurities analysis was run on both the Alliance System and the Arc HPLC System with a 5-µm particle size column using the conditions listed in Table 1. The Arc HPLC System successfully replicated the chromatographic separation acquired on the Alliance System, resulting in identical USP resolution between critical pair peaks 5 and 6 of 1.7 (Figure 1).
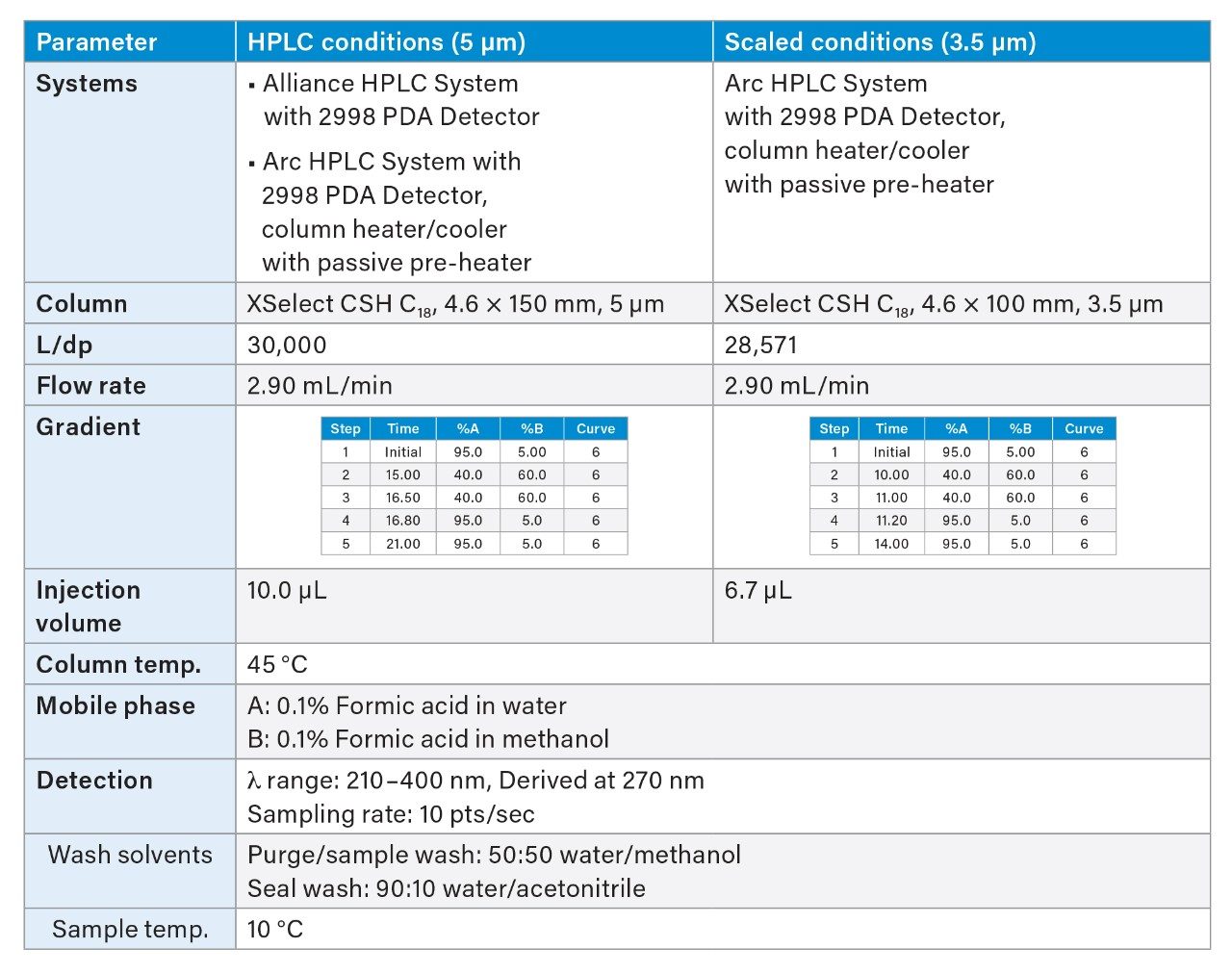
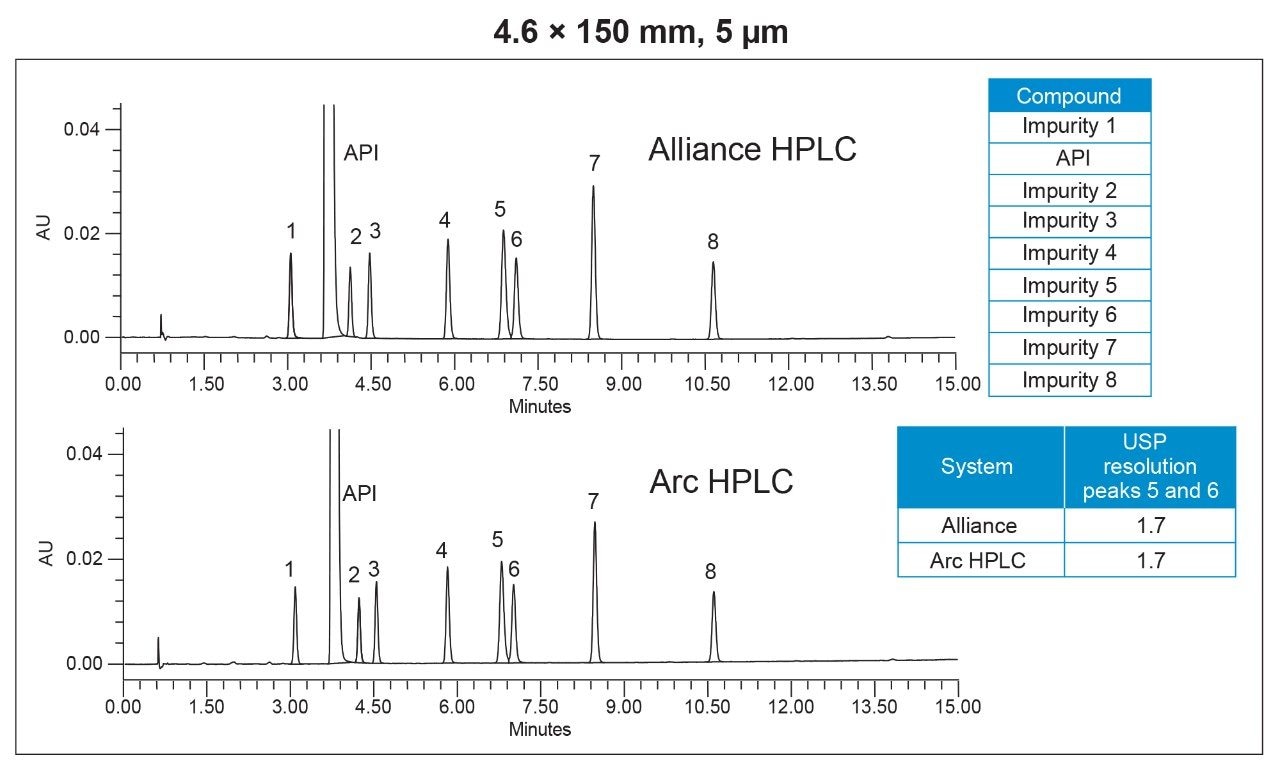
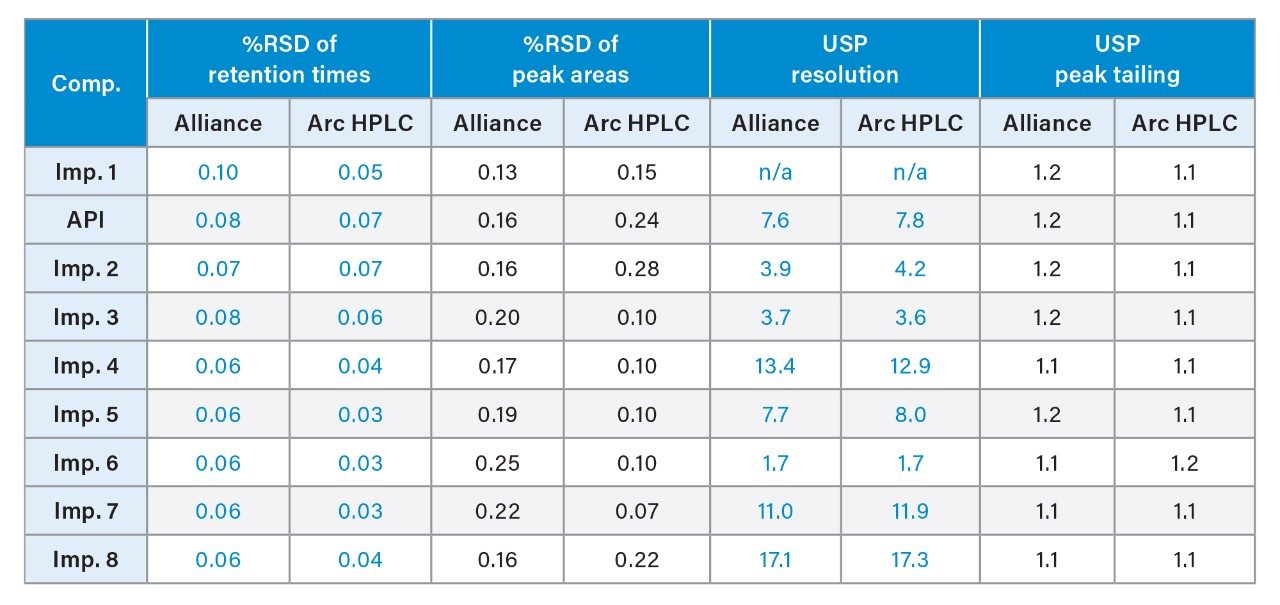
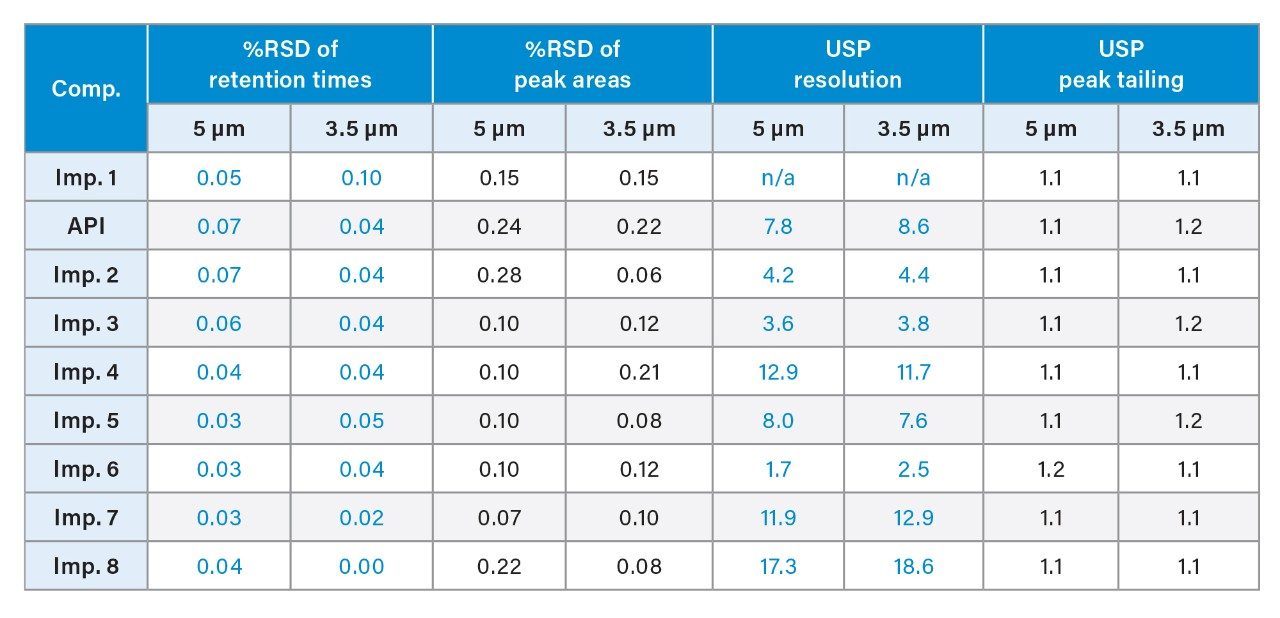
System suitability parameters, including relative standard deviation (RSD) of peak areas, and retention times, resolution, and peak tailing were used to assess chromatographic performance of the method run on the Arc HPLC System and compared to the data from the Alliance System. System suitability results from five replicate injections of a mixture containing impurities and the API is shown in Table 2. The repeatability of the retention times and peak areas were comparable on both systems. The RSD for peak areas on the Arc HPLC System was ≤0.28%, which is well below the USP specification of 2.0%.1 The USP resolution values between all the peaks were also comparable on both systems, indicating no loss in resolution for method transfer. The USP peak tailing factors were ≤1.2.
Transferring methods to the smaller particle columns reduces run times and solvent consumption, which can increase laboratory throughput and productivity. When scaling to a smaller particle, the ratio of column length (L) to particle size (dp) must be the same as the original method to preserve the resolving power of the method. Other parameters, including injection volume and gradient times, are scaled geometrically to maintain the same chromatographic separation quality and can be performed using the columns calculator.2 In this case, the flow rate was not geometrically scaled to reduce analysis run time.
In this study, the impurity method was scaled from a 5-µm to a 3.5-µm particle column with a shorter length, while keeping the same internal diameter (Table 1). Updating the method to a 3.5-µm particle column with the same flow rate of 2.9 mL/min reduced the run time by 33%, while maintaining the same separation integrity to that of a 5-µm method (Figure 2). The system suitability generated using 5 and 3.5 µm methods show comparable results for %RSD of retention times and peak areas, USP resolution, and peak tailing (Table 3).
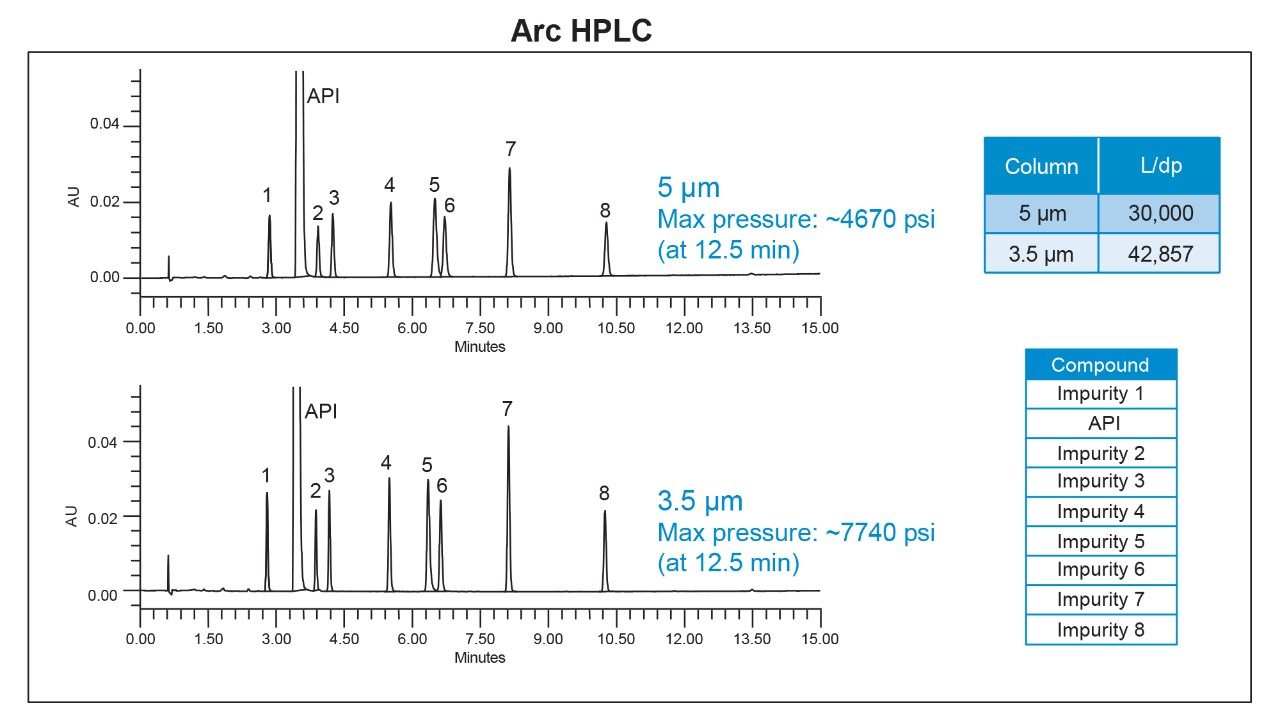
With modern LC instruments, improvements in engineering mean that traditional pressure limits no longer apply. With older LC instruments, scaling column dimension and particle size are critical to assure that the new method can be run within those lower pressure limits. The Arc HPLC System, can tolerate higher back pressures, which opens the opportunity to use higher resolving columns using the same analytical conditions.
Higher resolving power of the method can be achieved by increasing the L/dp ratio; that is keeping the same column length as the original method (L) but reducing the particle size (dp). However, moving to a smaller particle sized column with the same dimensions and conditions generally produces higher back pressure.
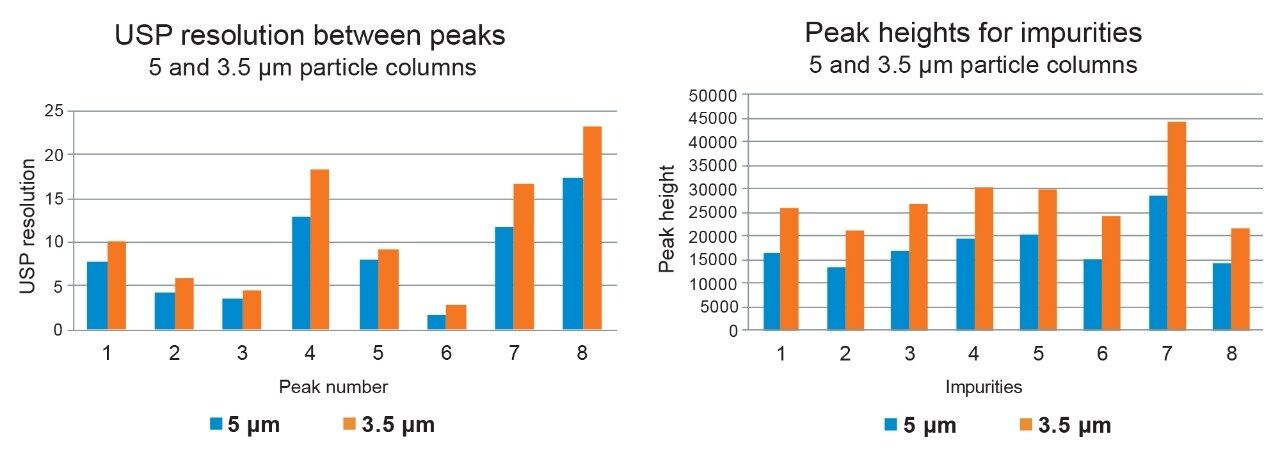
In case of the impurity method, it was repeated on the 4.6 x 150 mm column with 3.5-µm particle size using the same instrument conditions as for the 5-µm method (Table 1). The analysis performed on the 3.5-µm resulted in narrower and more efficient chromatographic peaks and produced higher back pressure (Figure 3). In addition, the 3.5-µm method resulted in significant improvement in the USP resolution between peaks and in peak heights compared to the 5-µm method (Figure 4), enabling easier and more robust integration for accurate quantification with increased sensitivity.
On a traditional LC system, this analysis would not be possible as the higher back pressure could not be tolerated. However, the Arc HPLC System, due to its high pressure limit, can accept methods with lower particle column when higher efficiency and extra resolving power is desirable.
The Arc HPLC System seamlessly accepts and replicates HPLC methods without compromising method integrity. It can also accommodate columns with 3.5 μm particles, either with scaled gradient steps for reduced run times and solvent consumption compared to the 5-µm method, or to take advantage of higher efficiency and extra resolving power that smaller particles offer when higher back pressure is not a limitation. Faster analytical runs and more robust peak integration improve laboratory efficiency and productivity for routine testing in QC laboratory.
The Arc HPLC System delivers robust, reliable, and reproducible performance and allows efficient method transfer from any LC platform, as well as effective method improvement. The high pressure limit of 9500 psi on the Arc HPLC System allows methods with high flow rates and smaller particles columns. Overall, this enables analytical laboratories to ensure product consistency and data quality, while minimizing data integrity concerns.
720006947, July 2020