This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates the use of IonHance Difluoroacetic Acid (DFA) as a mobile phase modifier for LC MS analysis of small molecules and compare it to formic acid and trifluoroacetic acid (TFA) modifiers.
IonHance DFA as a new choice to the limited number of suitable acidic mobile phase modifiers available for small molecule LC-MS analysis.
Mobile phase modifiers are key in LC-MS analysis, affecting chromatographic retention and peak width, as well as mass spectrometry (MS) signal response. Unlike LC with non-MS detectors, the choice of a suitable mobile phase modifier for LC with MS detection is limited. Additives used in LC-MS analyses must be sufficiently volatile, available in high purity, and able to give acceptable sensitivity. IonHance DFA meets these requirements, in that it is available at high purity with sodium and potassium levels below 100 ppb and is sufficiently volatile with a boiling point of 133.0 °C and vapor pressure of 1170 Pa.
IonHance DFA has been shown to be beneficial for LC-MS analyses of peptides and proteins, giving decreased peak widths relative to formic acid and increased MS sensitivity relative to TFA.1,2,3 Here, a comparison of IonHance DFA to formic acid and TFA is made for LC-MS analysis of acidic, basic, and neutral small molecules. The comparison is made in terms of chromatographic retention and peak width and MS signal response in both positive and negative electrospray ionization (ESI) modes.
Mobile phases were prepared by adding either IonHance DFA (p/n: 186009201), formic acid (Optima LC-MS grade, Fisher Chemical, p/n: A117-50) or TFA (Optima LC-MS grade, Fisher Chemical, p/n: A116-50) to a concentration of 0.1% (v/v) in both aqueous and acetonitrile mobile phases. The analytes listed in Table 1 with their optimized multiple reaction monitoring (MRM) transitions, were prepared at 2.5 μg/mL concentration in water and analyzed by separating them on an ACQUITY UPLC BEH C18, 1.7 μm, 2.1 x 50 mm Column using an ACQUITY UPLC I-Class System with a Xevo TQ-S MS/MS. Chromatographic retention, peak widths, and MS signal response were measured under acetonitrile gradient conditions (5–100%). Since the aqueous/organic ratio in the mobile phase can impact the MS signal response, two probe analytes, 2,6-dimethylaniline and 4-chloro-Nmethylaniline, were also analyzed by MS via post LC infusion at different aqueous/organic ratios to compare the MS signal response obtained using the three additives at fixed aqueous/organic compositions.
Figure 1 shows a comparison of the retention times for all the analytes using the three mobile phase modifiers. While the retention times of the neutral analyte 2-chloro-4-nitroaniline were similar for the three modifiers, the retention times of the other compounds, which are ionized, showed significant differences. The aqueous modifier solutions vary in pH from 2.0 (0.1% v/v TFA and 0.1% DFA) to 2.7 (0.1% v/v formic acid), and this affects the retention times of analytes that have pKa values in the 1–4 range. For the compounds that have a positive charge under the separation conditions, differences in the hydrophobicity of the modifiers also affect the retention times because the anion of the modifier ion-pairs with positively-charged analytes. TFA has the greatest hydrophobicity and formic acid the least. Similar retention time differences have been reported for peptides.2
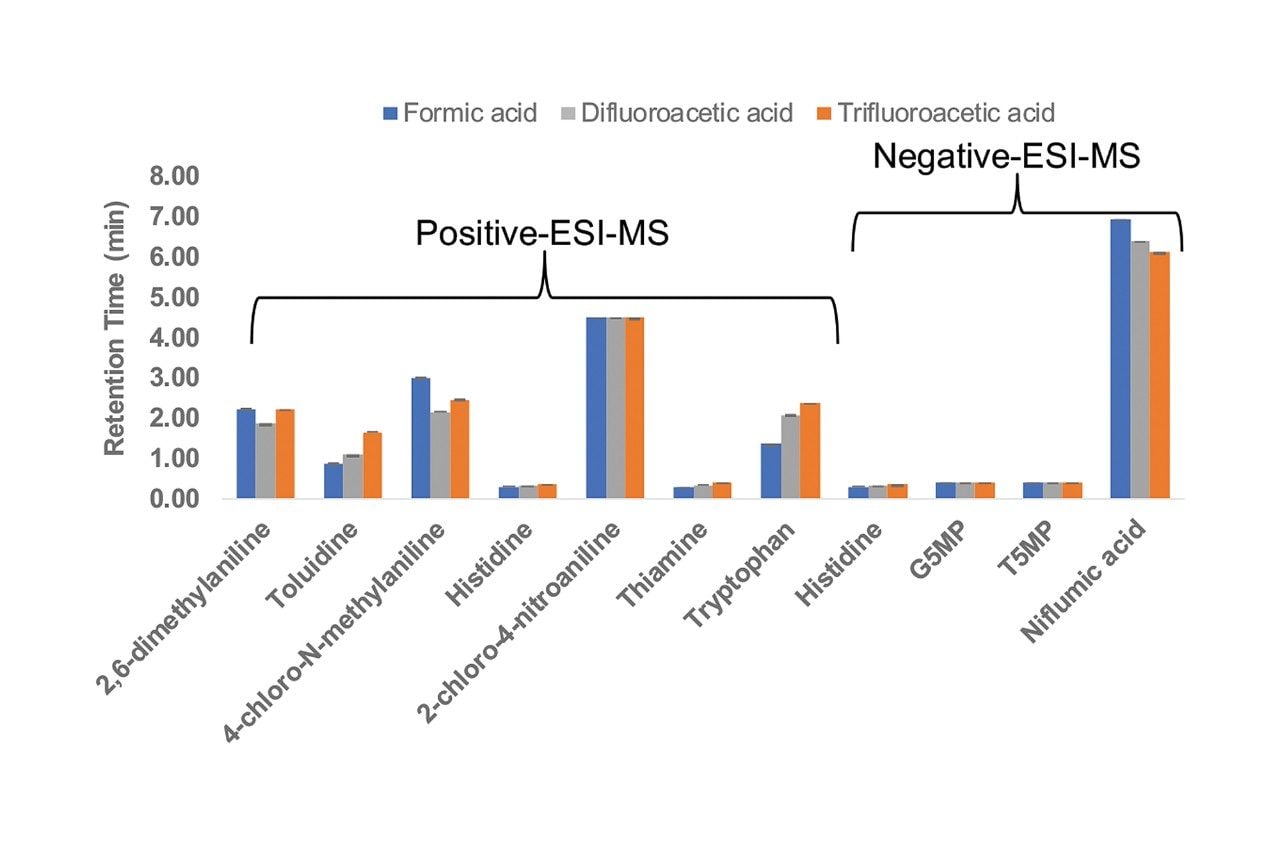
Figure 2 shows a comparison of the chromatographic peak widths for all the analytes using the three mobile phase modifiers. For most of the compounds, the peak widths obtained using DFA are smaller than those obtained using formic acid and similar to those obtained using TFA. The same trend has been reported for peptides.1,2
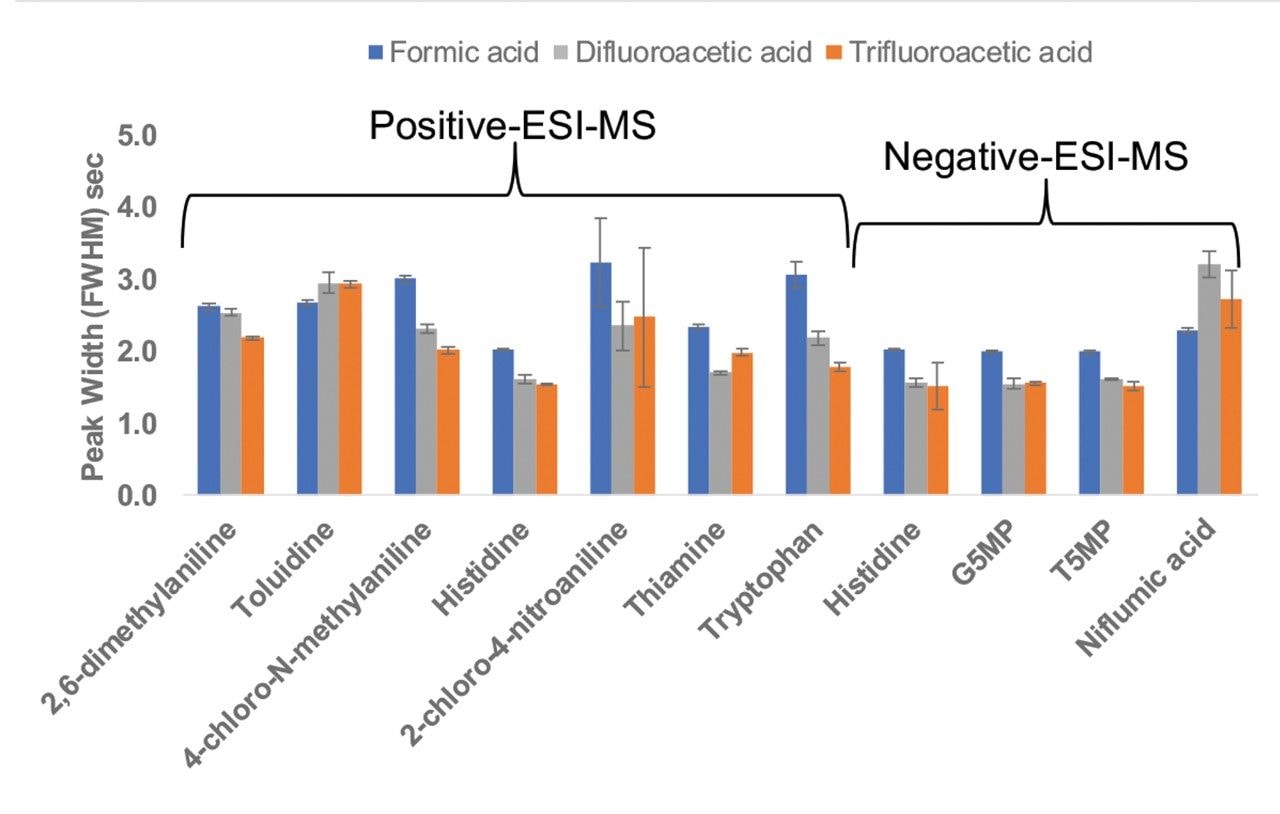
Figure 3 shows the MS signal response (peak area) for all the analytes under the same LC-MS conditions using the three mobile phase modifiers. For all the analytes, MS signal response using DFA was significantly higher (up to two-fold in magnitude) when compared to TFA. For acidic analytes the MS signal response when using DFA was comparable to the response using formic acid. Most of the basic analytes showed improved MS signal response using DFA compared to formic acid. Previous studies using peptide analytes showed similar trends.1,2
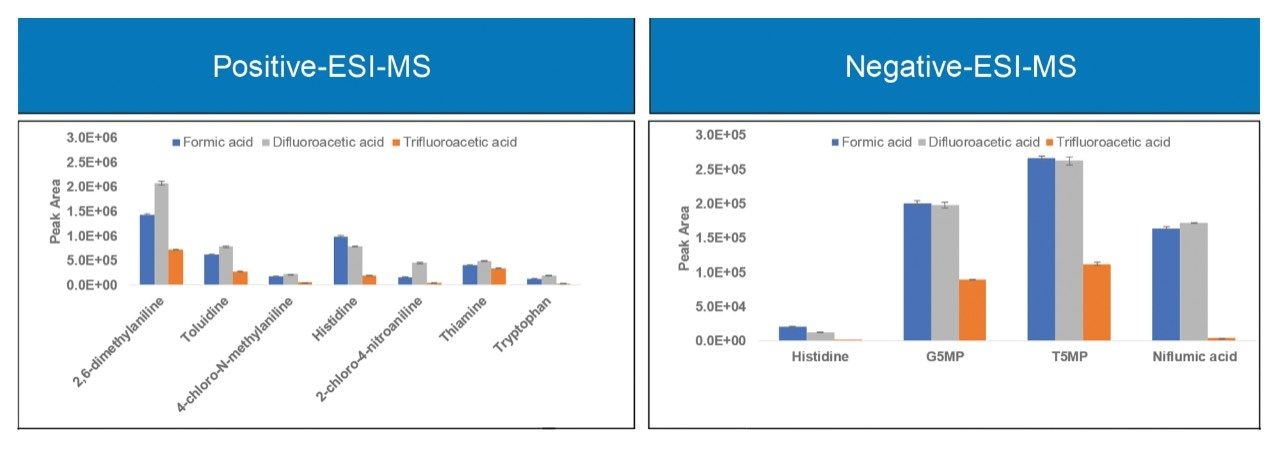
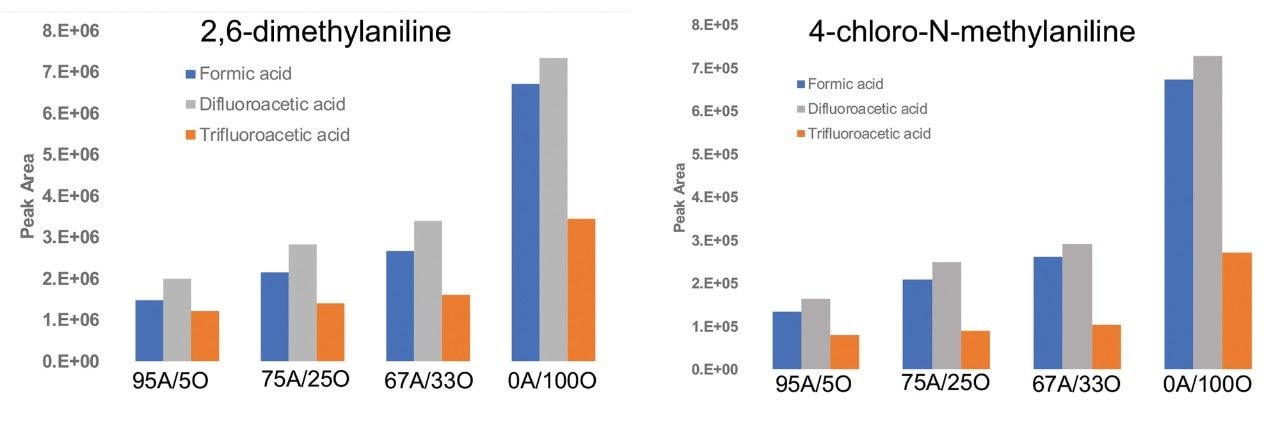
Figure 4 shows the MS signal response for two of the basic analytes, 2,6-dimethylaniline and 4-chloro-N-methylaniline at fixed aqueous/organic mobile phase compositions. It is evident from the results that the MS signal response for these analytes is slightly higher using IonHance DFA compared to formic acid and is significantly higher when compared to TFA at different aqueous/organic mobile phase compositions.
IonHance DFA shows great potential for use as a mobile phase modifier in small molecule LC-MS analysis, adding a new choice to the limited number of suitable acidic modifiers. For the analytes tested, IonHance DFA exhibits the combined benefits of formic acid and TFA modifiers, giving narrow peak widths comparable to those obtained using TFA and high MS signal responses like those obtained using formic acid.
720006776, March 2020