
This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates a highly sensitive and robust LC-MS/MS quantification method for six nitrosamine impurities (NDMA, NDEA, NEIPA, NDIPA, NDBA, and NMBA) in solutions containing irbesartan, losartan, valsartan (sartans), and ranitidine drug substances.
The Xevo TQ-XS Mass Spectrometer, coupled to an ACQUITY UPLC I-Class PLUS System with an HSS T3 Column for separation, enables high sensitivity quantification of nitrosamine impurities from ranitidine and sartan drug substances, achieving LLOQs of 0.1 ng/mL.
N-nitroso compounds are considered to have extremely high carcinogenic potency, and several medications have been subject to recalls due to the presence of these impurities.1,2 To ensure the safety of pharmaceutical products, steps must be taken to understand the source of these impurities and to ensure their removal from the final drug substance. Information on how to assess and control these carcinogenic impurities can be found in the ICH M7(R1) guideline.3
Here, we present a robust and highly sensitive LC-MS/MS method for simultaneous quantification of six nitrosamine impurities (NDMA, NDEA, NEIPA, NDIPA, NDBA, and NMBA). This method affords 0.1 ng/mL LLOQs (3 pg on-column) with a linear dynamic range from 0.1–100 ng/mL.
|
LC conditions |
|
|---|---|
|
LC system: |
ACQUITY UPLC I-Class PLUS, FTN with 50 μL extension loop |
|
Column: |
HSS T3; 1.8 μm, 100Å, 2.1 x 100 mm |
|
Column temp.: |
40 °C |
|
Sample temp.: |
10 °C |
|
Injection volume: |
30 μL |
|
Mobile phase: |
A – 5 mM ammonium formate with 0.1% formic acid in water B – 5 mM ammonium formate with 0.1% formic acid in methanol |
|
Purge solvent: |
50:50 water:methanol |
|
Wash solvent: |
25:25:25:25 IPA: MeOH:ACN:water |
|
Sample diluent: |
Water |
|
Time (min) |
Flow (mL/min) |
%A |
%B |
Curve |
|---|---|---|---|---|
|
Initial |
0.400 |
98.0 |
2.0 |
Initial |
|
0.24 |
0.400 |
98.0 |
2.0 |
6 |
|
4.00 |
0.400 |
5.0 |
95.0 |
6 |
|
4.61 |
0.400 |
5.0 |
95.0 |
6 |
|
5.00 |
0.400 |
98.0 |
2.0 |
6 |
|
7.00 |
0.400 |
98.0 |
2.0 |
6 |
|
MS conditions |
|
|---|---|
|
Source: |
APCI+ |
|
Corona: |
0.5–1.3 μA (system/pin specific) |
|
APCI probe temp.: |
400 °C |
|
Desolvation flow: |
1000 L/Hr |
|
Cone gas flow: |
150 L/Hr |
|
Data management: |
Chromatography software: MassLynx Quantification software: TargetLynx XS |
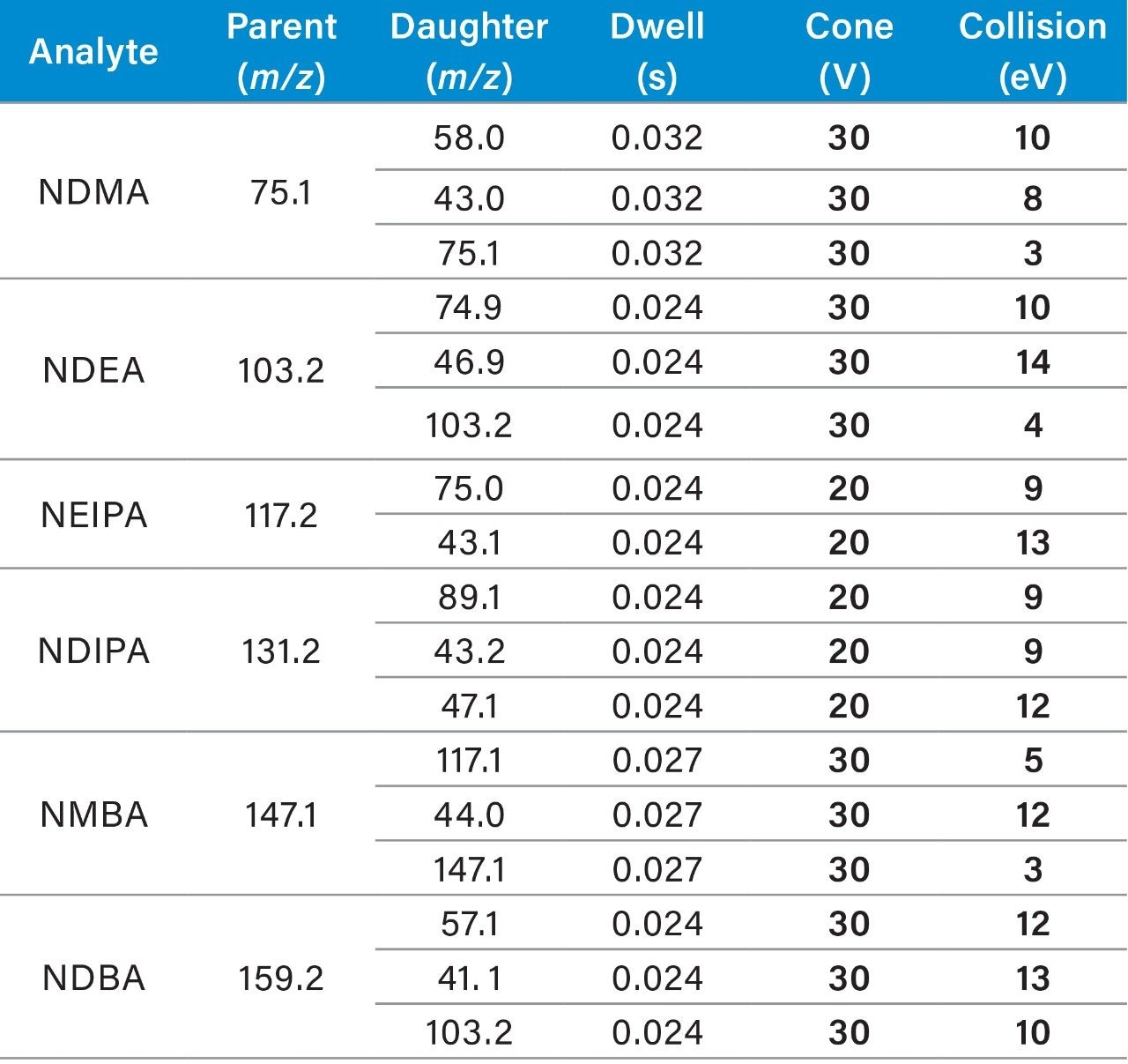
Standard samples containing the six nitrosamine impurities and four drug substances (ranitidine and sartans) were prepared from a concentrated stock solution containing 1 μg/mL of each nitrosamine impurity and ~100 μg/mL of each drug substance in 20% methanol/80% water. Calibration standards (0.05–100 ng/mL) were prepared in water by serial dilution from the 1 μg/mL solution and placed into a 96-well sample collection plate (p/n 186005837). The plate was sealed using a pre-slit Silicone/PTFE 96-well cap mat (p/n 186006332). LC-MS conditions are listed in Table 1, while the specific MRM transitions for the nitrosamines and drug products used for analysis are listed in Table 2. LC-MS/MS analysis was performed using a Waters Xevo TQ-XS Tandem Quadrupole mass spectrometer coupled to an ACQUITY UPLC I-Class PLUS System. Chromatographic separation of the nitrosamines and drug substances was achieved with an ACQUITY HSS T3 Column, based on a previous method.4 Due to its unique polar chemistry, the ACQUITY HSS T3 Column provided excellent retentivity for the nitrosamines, particularly retention of the most polar nitrosamine, NDMA (Figure 1). The Xevo TQ-XS MS, featuring a novel StepWave ion guide and IonSABRE APCI probe, improved ion sampling in the source, enabled efficient ion transfer, and enhanced ionization. MassLynx (v4.2) and TargetLynx XS chromatographic and data processing software were used for data acquisition and quantification. The same quantitative functionality is also available in Waters’ compliant-ready solution, MassLynx Security. This quantification performance is highlighted in Table 3, while chromatographic performance, highlighting the LLOQs of the six nitrosamine impurities, is illustrated in Figure 2. With this developed assay, LLOQs of 0.1 ng/mL were achieved, with accuracies and RSDs ≤15%, demonstrating a highly sensitive, accurate, and robust method for nitrosamine impurity quantification.
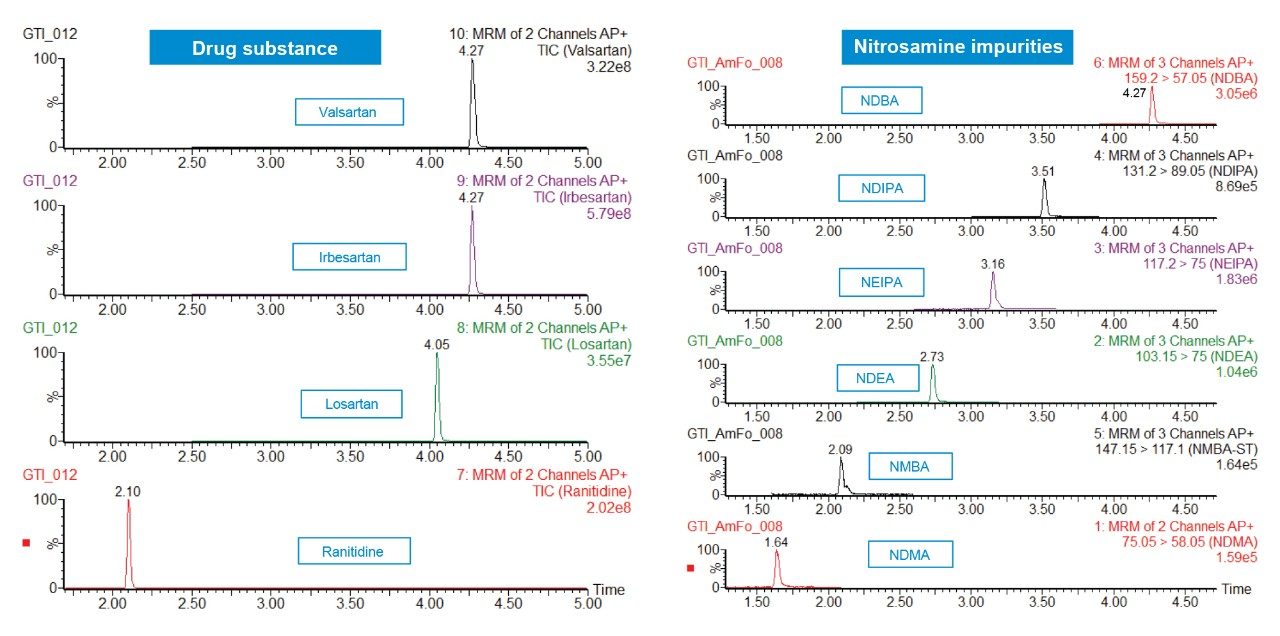
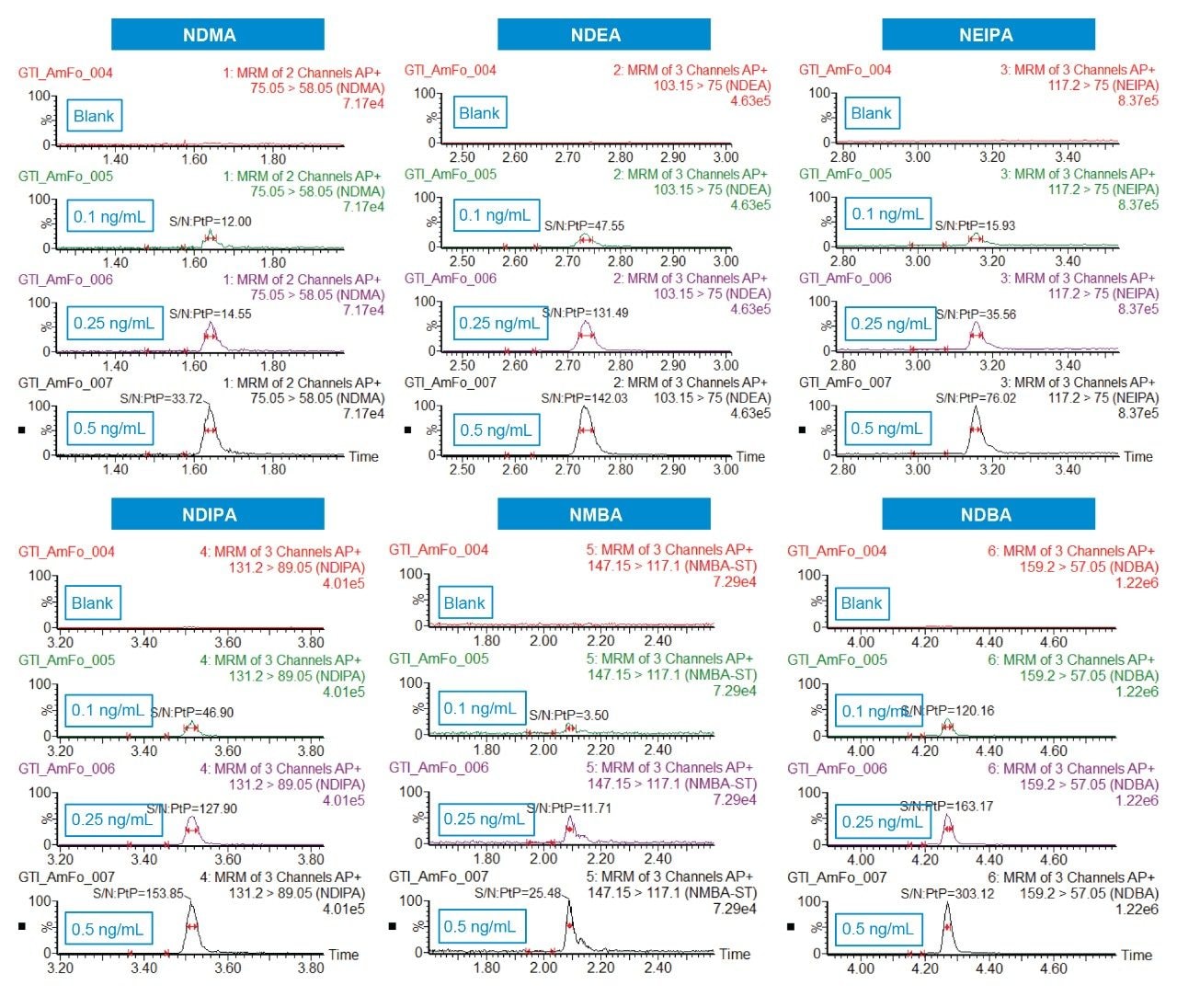
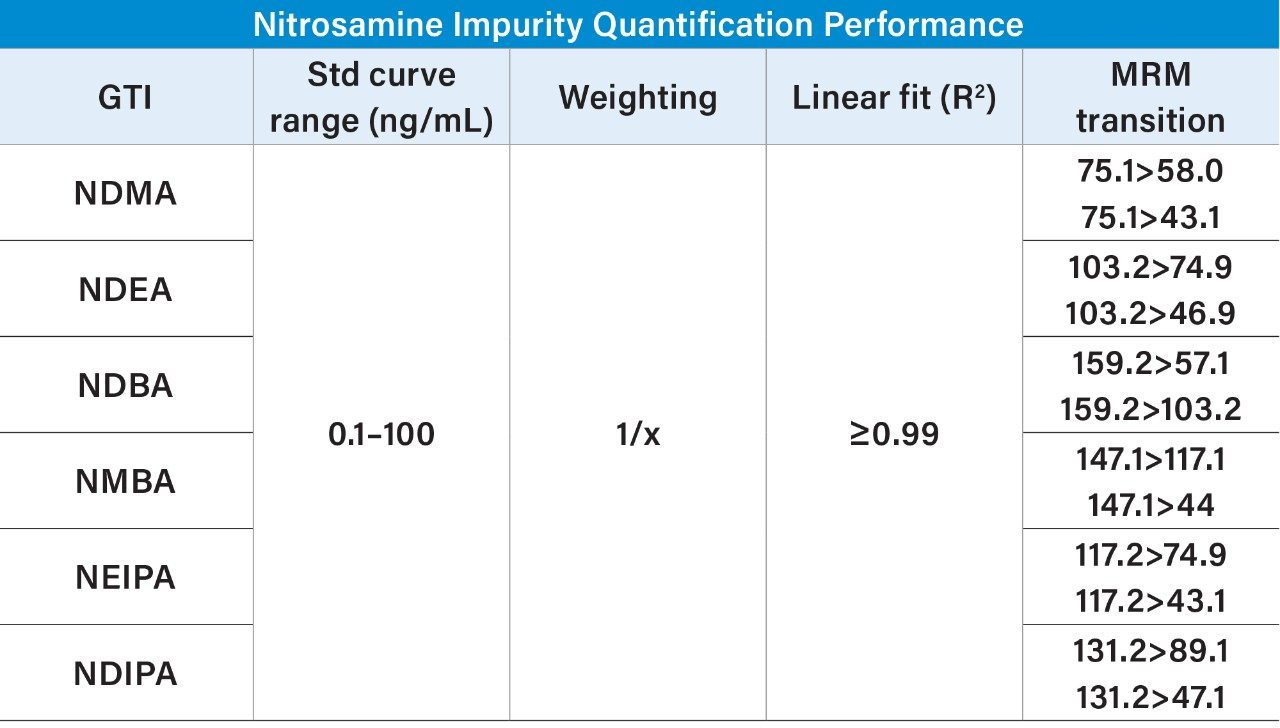
A single UPLC-MS/MS method was successfully developed for the accurate, robust, and highly sensitive quantification of six nitrosamine impurities, achieving LLOQs of 0.1 ng/mL using the ACQUITY UPLC I-Class PLUS and the Xevo TQ-XS Tandem Quadrupole Mass Spectrometer. The ACQUITY HSS T3 Column provided excellent retentivity and selectivity for six nitrosamine impurities. This method offers a practical starting point for high sensitivity quantification of nitrosamines or similar compounds.
720006751, January 2020