This is an Application Brief and does not contain a detailed Experimental section.
This application brief demonstrates quick and accurate analysis of NDMA in ranitidine drug substance and drug product by UV detection, with the added benefit of mass confirmation by mass spectral data using an ACQUITY QDa Mass Detector.
The ACQUITY Arc System with PDA Detector, integrated with an ACQUITY QDa Mass Detector, enables accurate analysis of NDMA in ranitidine drug substance and drug product.
N-nitrosodimethylamine (NDMA) is classified as a probable human carcinogen, a compound that can cause DNA mutations, potentially leading to cancer.1 The ICH M7 recommends that known mutagenic carcinogens, such as nitrosamines, should be controlled at or below the acceptable cancer risk level to ensure safety of the pharmaceutical products.
Since July 2018, several prescription and over-the-counter medications containing ranitidine were found to contain NDMA.2 Since then, the industry has been working to develop suitable analytical methods to accurately measure levels of this impurity in pharmaceutical products at all stages of the development and manufacturing process, as well as in final API and drug products. The ACQUITY Arc System with PDA Detector, integrated with an ACQUITY QDa Mass Detector, enables accurate analysis of NDMA in ranitidine drug substance and drug product.
In this work, we present an HPLC method with UV detection for quick and accurate analysis of NDMA in ranitidine drug substance and drug tablet formulation using an ACQUITY Arc System with PDA Detector coupled to an ACQUITY QDa Mass Detector. In combination, the UV data is used for the determination of NDMA in test sample solutions, while the mass spectral data from the ACQUITY QDa Mass Detector enables quick and accurate peak identity confirmation.
|
Parameter |
Description |
|---|---|
|
LC systems: |
ACQUITY Arc System with 2998 PDA and ACQUITY QDa detectors, passive pre-heater, and flow path 1 |
|
Column: |
XSelect HSS T3 4.6 x 100 mm, 3.5 μm |
|
Column temp.: |
40 °C |
|
Flow rate: |
1.0 mL/min |
|
Injection volume: |
25.0 μL |
|
Mobile phase: |
A: water with 0.02% of formic acid B: acetonitrile |
|
Wash solvents: |
Purge: 50:50 water/acetonitrile Sample wash: 80:20 water/methanol Seal wash: 90:10 water/acetonitrile |
|
PDA detection: |
γ range: 210–400 nm, Derived at 245 nm Sampling rate: 10 pts/sec |
|
Mass detection: |
ACQUITY QDa Detector Ionization mode: ESI+ Acquisition range: 50–500 m/z Diverter valve: switch flow to waste at 2.70 minutes |
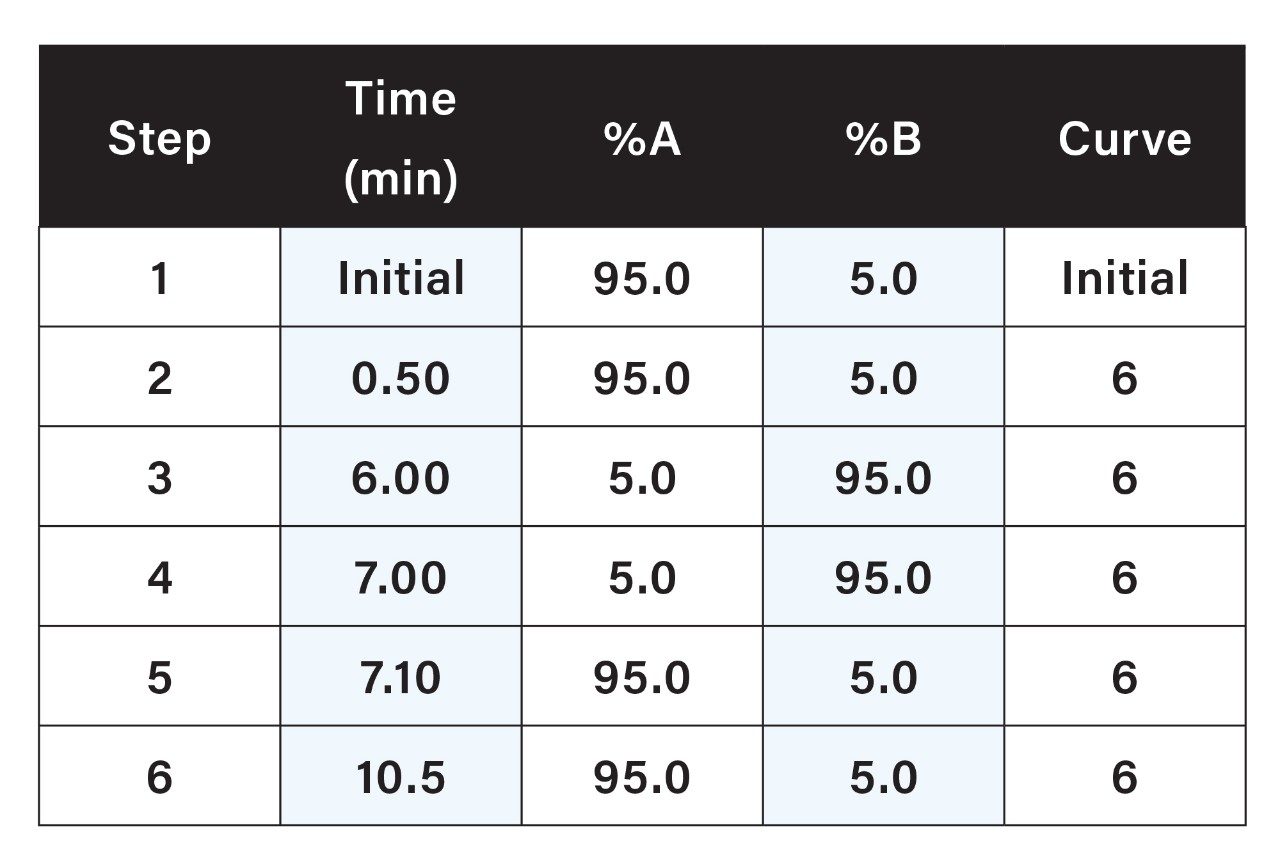
Ranitidine drug substance test samples were prepared in water at 30 mg/mL. Drug product tablet formulations were prepared by dissolving crushed tablets in water to contain 30 mg/mL of ranitidine. After extraction, sample test solutions were centrifuged for 30 minutes at 4000 rpm and filtered using 0.2 um PVDF syringe filters. NDMA commercially available stock (Sigma P/N CRM40059) was diluted in 20% MeOH to 250 μg/mL. This solution was subsequently diluted with water to make NDMA calibration standards ranging from 10 to 300 ng/mL.
The HPLC separation was performed using an XSelect HSS T3 Column with the conditions described in Table 1. The method provided excellent retention of NDMA and separation from the ranitidine peak (Figure 1).
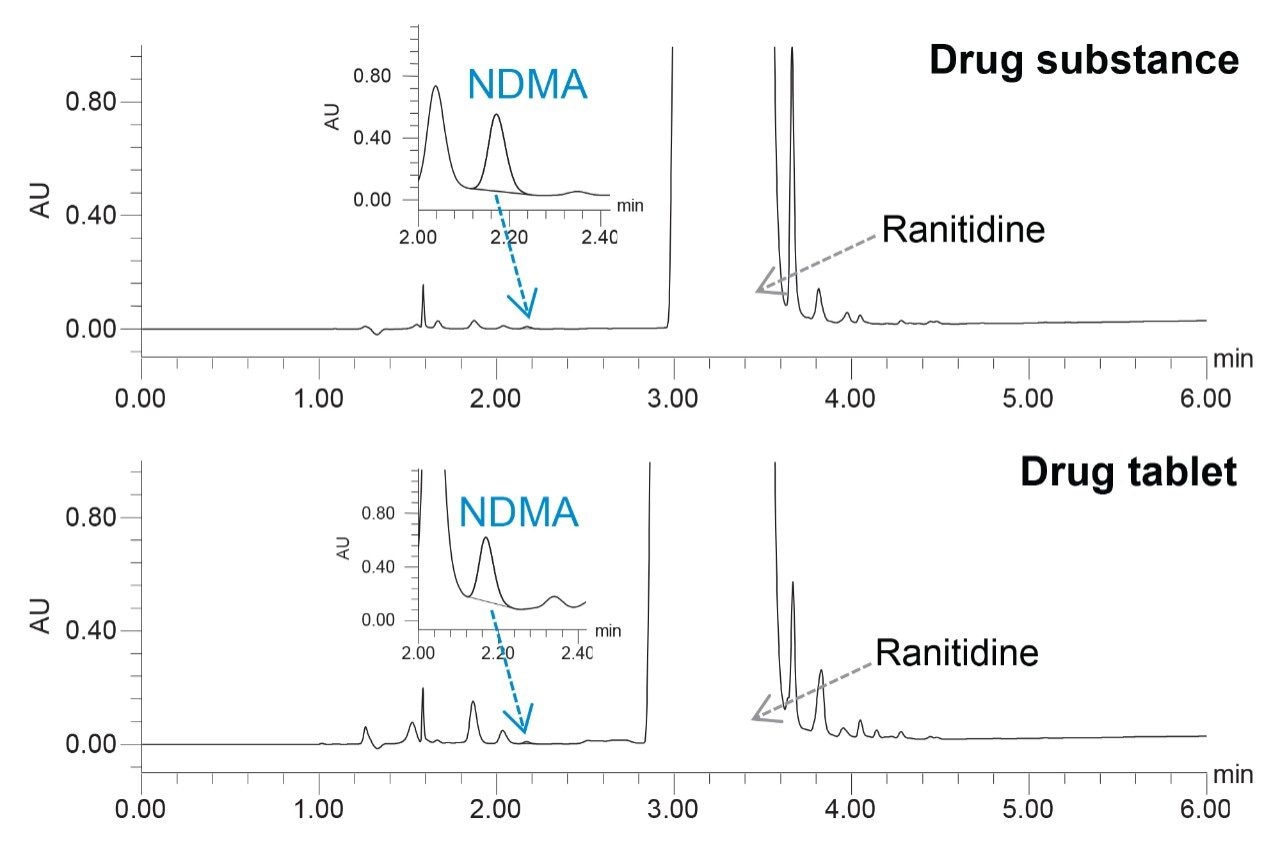
The analysis of drug substance and drug tablet test sample solutions prepared at 30 mg/mL of ranitidine showed presence of NDMA impurity (Figure 2). The age, expiration and storage conditions of the ranitidine drug substance and drug product was unknown. Both the drug substance and drug product test samples were spiked with the NDMA standard to evaluate accuracy in the matrix.
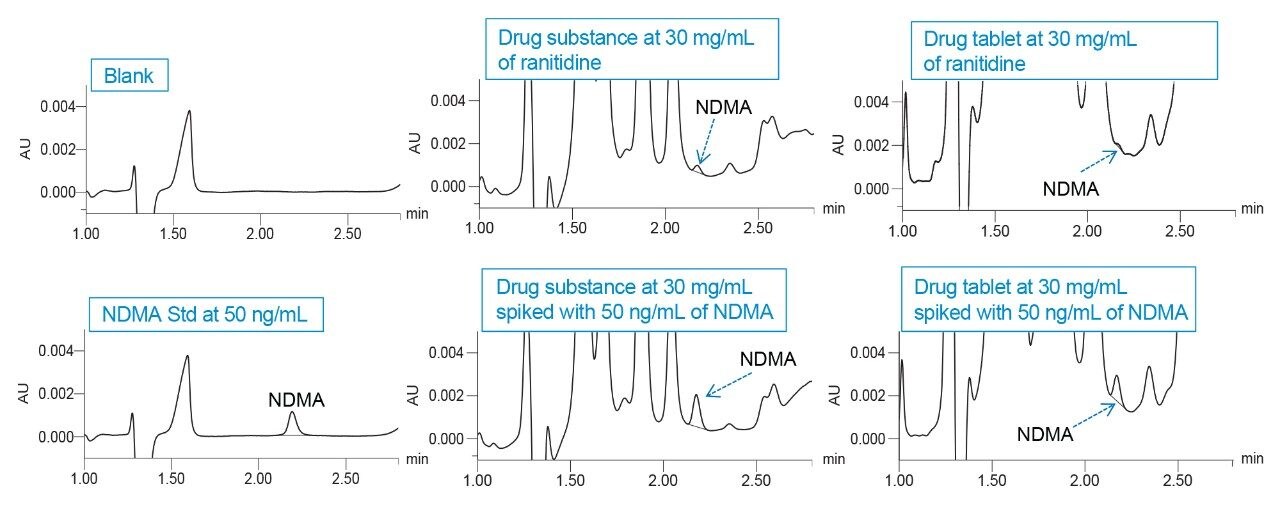
The % recovery of NDMA spiked into the test samples was calculated by subtracting the already present amount of NDMA in the test samples and quantified against a calibration curve. The average % recovery of 50 ng/mL NDMA spiked into the test sample solutions was 85%.
Previously reported UV quantitation limits of nitrosamine impurities determined in valsartan and ranitidine drug substance are summarized in Waters Tech Brief 720006775EN.3
The HPLC/UV method enabled quick and accurate analysis of NDMA in ranitidine drug substance and drug tablet products. The analysis was performed on the ACQUITY Arc System with 2998 PDA Detector, integrated with an ACQUITY QDa Mass Detector for quick and accurate peak identity confirmation. The XSelect HSS T3 Column, due to its unique polar chemistry, provided excellent retentivity for a polar nitrosamine impurity, NDMA. Overall, this method provides a quick and easy solution for the accurate determination of NDMA in medications containing ranitidine.
720006898, June 2020