This is an Application Brief and does not contain a detailed Experimental section.
Analyte/surface adsorption in liquid chromatography (LC) is a contributing factor in poor peak shape, tailing, and diminished recovery of compounds in LC-based techniques. In this study, the performance of the ACQUITY Premier Solution featuring MaxPeak High Performance Surfaces (HPS) was evaluated in its ability to mitigate metal-induced adsorption phenomena of an “acidic” peptide (sequence: VDNALQSGNSQESVTEQDSK, pI =3.9). Using a RPLC/MS-based technique, up to a 34-fold increase in MS detector response was observed for the acidic peptide. This was in part attributed to the significant reduction in tailing observed when using the ACQUITY Premier MaxPeak HPS technology in comparison to conventional hardware. Furthermore, up to a 4-fold increase in peak area was observed when using the ACQUITY Premier LC System and Column demonstrating the MaxPeak HPS technology also improved the recovery of the metal sensitive peptide. The chromatographic performance gains observed with ACQUITY Premier Solution featuring MaxPeak HPS technology resulted in a 3-fold increase in high-energy b/y fragment ions (11 vs. 36) when compared to the conventional LC system and column. In summary, this study demonstrates how the chromatographic performance gains observed with ACQUITY Premier featuring MaxPeak HPS technology can increase productivity in the lab and improve data quality for metal-sensitive analytes.
The ACQUITY Premier Solution significantly improves recovery, peak shape, and reproducibility of peptides containing acidic amino acid residues compared to stainless-steel hardware
The analysis of biomolecules on conventional stainless-steel LC systems and columns has demonstrated that non-specific adsorption contributes to reduced sample recovery and peak tailing of sensitive analytes leading to increased assay variability and reduced detector response. In certain instances, these losses can be attributed to undesirable interaction of analytes with metal surfaces. The underlying mechanism being that analytes bearing electron-rich moieties acts as a Lewis base and adsorb in a non-covalent manner to electron deficient active sites on the metal surface of the LC and column hardware. Acidic peptides, or peptides that exhibit a net negative charge at neutral pH due to the presence of acidic amino acids (e.g. aspartic acid or glutamic acid), are particularly susceptible to this adsorption phenomena. Given that amino acids ubiquitously serve as the building blocks of protein-based therapeutics, challenges associated with metal-induced adsorption phenomena are frequently encountered in the development and manufacturing of biopharmaceuticals. Efforts to reduce risk and untimely delays associated with method development or routine investigations require deployable solutions that can deliver reproducible and robust results for peptide-based assays.
The ACQUITY Premier Solution featuring MaxPeak High Performance Surface (HPS) technology is the answer to challenges associated with adsorptive losses due to analyte/surface interaction. Based on experience and established knowledge, the MaxPeak Premier product line featuring MaxPeak HPS technology is purposely engineered with a barrier layer to reduce non-specific adsorption of analytes for increased recovery, improved peak shape, and reproducibility of sensitive analytes. The objective of this study is to demonstrate the benefits of the ACQUITY Premier Solution in the analysis of an “acidic” peptide using a reversed-phase liquid chromatography technique to perform a peptide mapping assay typical of industry.
A comparison of three system configurations was made to examine how ACQUITY Premier featuring MaxPeak HPS technology improves sample recovery and peak shape compared to systems configured with conventional metal surfaces. These configurations include: a conventional LC system with a stainless-steel column, the same conventional LC system configured with an ACQUITY Premier Column, and the ACQUITY Premier Solution (comprised of an ACQUITY Premier System configured with an ACQUITY Premier Column featuring MaxPeak HPS technology). In this study, the T14 peptide (sequence: VDNALQSGNSQESVTEQDSK) from the enzymatically digested NIST mAb reference material (Waters P/N: 186009126) was used to evaluate the performance of ACQUITY Premier with MaxPeak HPS technology. The T14 peptide is well suited for this evaluation given its acidic composition (20 %, bold annotation) and known susceptibility towards metal-induced surface adsorption. Samples were run under reversed phase LC conditions using a 0.68% B/min gradient (MP A: H2O, 0.1 % v/v FA, MP B: MeCN, 0.1 % v/v FA). A SYNAPT XS HRMS was used for data acquisition (see figure for instrument settings) with data processing and peptide identification performed using Waters UNIFI Scientific Information System (v1.9.4). An ACQUITY UPLC H-Class Bio Binary PLUS System configured with an ACQUITY UPLC CSH C18 column (P/N: 186005297) was used to represent a conventional LC system for comparison to the ACQUITY Premier Solution which was comprised of a ACQUITY Premier LC System configured with an ACQUITY Premier CSH Column (P/N: 186009488).
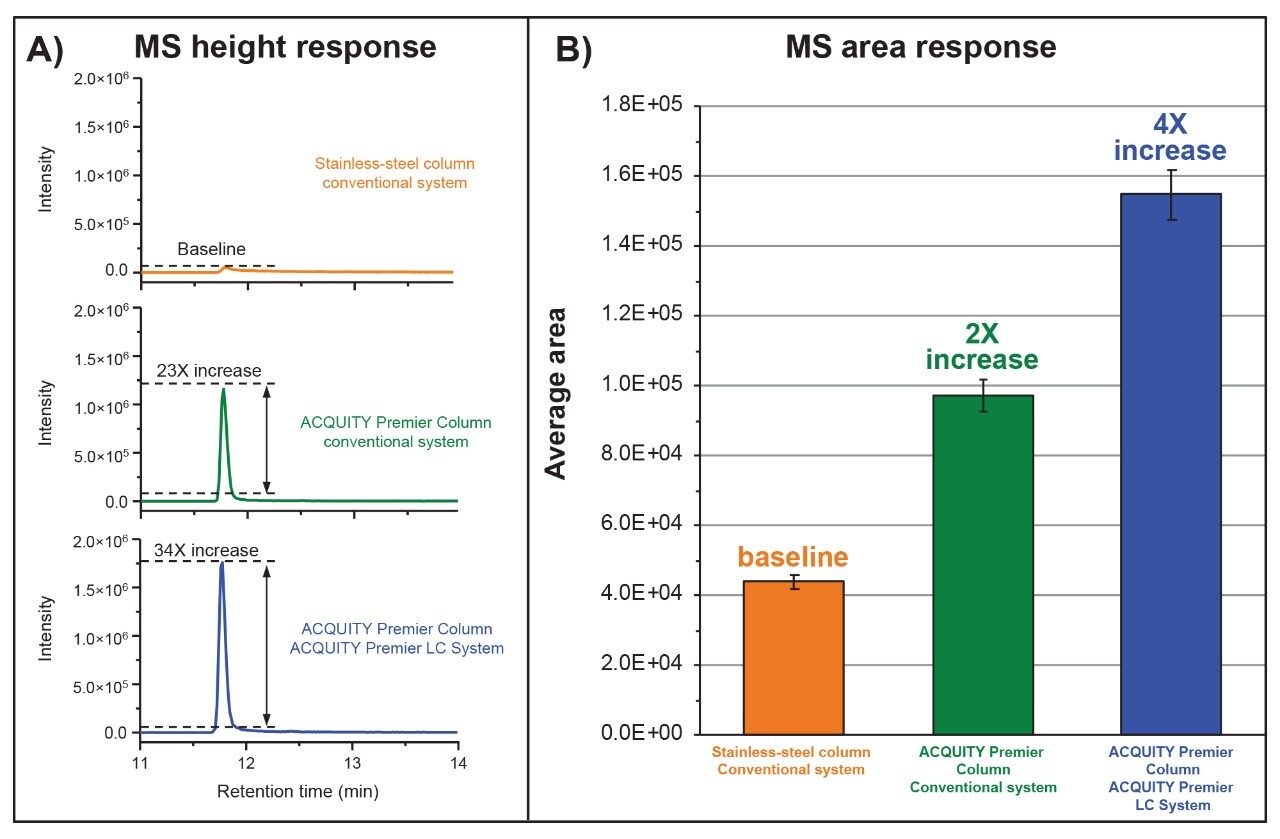
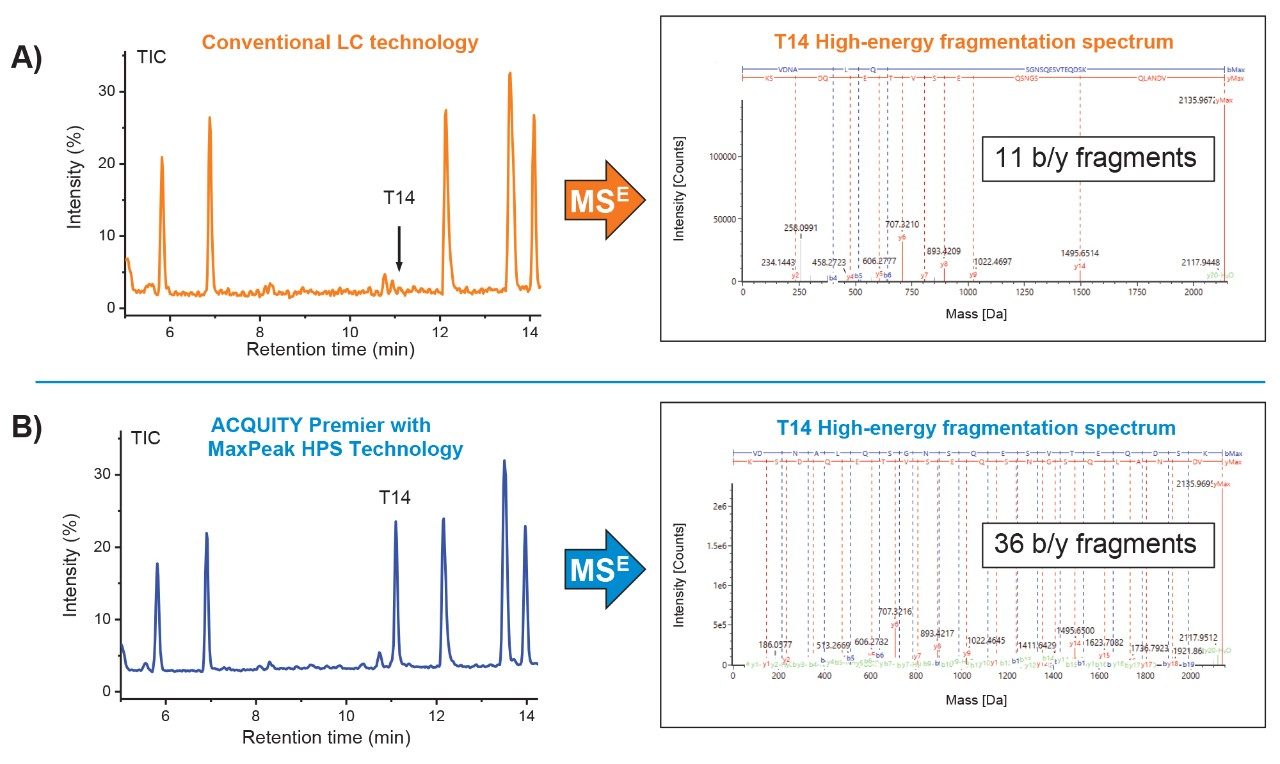
As shown in Figure 1A, the T14 peptide exhibited significant tailing resulting in severe peak broadening with a maximum signal observed at 5.2×104 ion counts when the peptide map was performed on the conventional LC configured with a stainless-steel column (orange trace). In contrast, tailing and peak shape were significantly improved when performing the same separation on the Waters ACQUITY Premier Column featuring MaxPeak HPS technology resulting in a 23-fold increase in signal intensity with maximum signal response of 1.2×106 ion counts (green trace). More notable was when the ACQUITY Premier Solution (ACQUITY Premier System configured with an ACQUITY Premier Column) was used for the separation the instrument response improved 34-fold (ion counts =1.8×106) when compared to the conventional system using a stainless-steel column as shown in the blue trace of Figure 1A. The improved performance observed when using ACQUITY Premier technology with MaxPeak High Performance Surfaces is attributed to its ability to not only reduce metal-induced adsorption artifacts, such as tailing for sensitive analytes, but improve recovery of analytes. This is demonstrated in the increasing area response of each experiment as shown in Figure 1B. In this instance, the ACQUITY Premier Solution facilitated up to a 4-fold improvement in peptide recovery which would otherwise be lost to the metal surface of the system and column, demonstrating the combined benefits of MaxPeak HPS technology when deployed as an integrated solution in both the LC system and column hardware. The performance gains observed with ACQUITY Premier technology featuring MaxPeak HPS directly translates to improved data quality as demonstrated in Figure 2. Using modest filtering parameters, only 11 b/y fragment ions were detected during data independent acquisition (MSE) analysis of the T14 peptide when using the conventional system configured with the stainless-steel column (Figure 2A). In contrast 36 b/y ions were detected for the T14 peptide when using ACQUITY Premier with MaxPeak HPS technology (Figure 2B) for improved confidence in data interpretation and peptide assignment. Collectively, these examples clearly demonstrate the value ACQUITY Premier Solution with MaxPeak HPS technology brings to the lab for improved productivity and confidence in the analysis and interpretation of data.
Non-specific adsorption of analytes when using conventional LC systems configured with stainless-steel hardware can lead to reduced sample recovery and poor peak shape. The Waters ACQUITY Premier Solution featuring MaxPeak High Performance Surfaces addresses the challenges associated with non-specific adsorption resulting in improved recovery, peak shape, and reproducibility of sensitive analytes. The benefits of ACQUITY Premier with MaxPeak HPS technology enable scientists to increase productivity in the lab and mitigate risk through increased reproducibility, recovery, and robustness of assays performed in the development and manufacturing of biopharmaceutical drug products.
720007173, March 2021