Routine Accurate Mass Measurement for the Identification of Impurities in Quetiapine Fumarate API Using the SmartMS-Enabled ACQUITY RDa Detector
Abstract
This application note demonstrates a simplified workflow for routine accurate mass measurement for the identification of Quetiapine and its related impurities with the ACQUITY RDa Detector. The full scan with fragmentation function provides simultaneous acquisition of both low and high energy data, maximizing the information gathered from a single injection. The UNIFI Application Workflow streamlines the fragment analysis and structural elucidation process and provides greater confidence in the end results. This workflow-based approach delivers rapid comprehensive results for identification and confirmation of impurities in Quetiapine fumarate API (active pharmaceutical ingredient). The API and its related impurities were identified and reported using the UNIFI application within the waters_connect Software platform. The full scan with fragmentation function of ACQUITY RDa Detector generated fragment ion information. By ramping the fragmentation cone voltage additional structural information was generated.
Knowledge of the impurity profile of a drug is vital as the chemical degradation of its active component often results in a loss of potency, affecting its efficacy and safety; therefore, it is important to study its stability using an appropriate analytical tool such as high-resolution mass spectrometry (HRMS). The ACQUITY RDa Detector, with its automatic set up and calibration, allows accurate mass measurements to be obtained by scientists with a diverse range of analytical expertise. Thus, providing access to HRMS for non-expert users and empowering scientists with a far greater depth of analytical information.

Benefits
- Routine accurate mass measurements for the impurity profiling
- Compact benchtop system with SmartMS Technology
- Intuitive system health checks and dedicated end-to-end workflows
- Compliant ready system with data integrity
Introduction
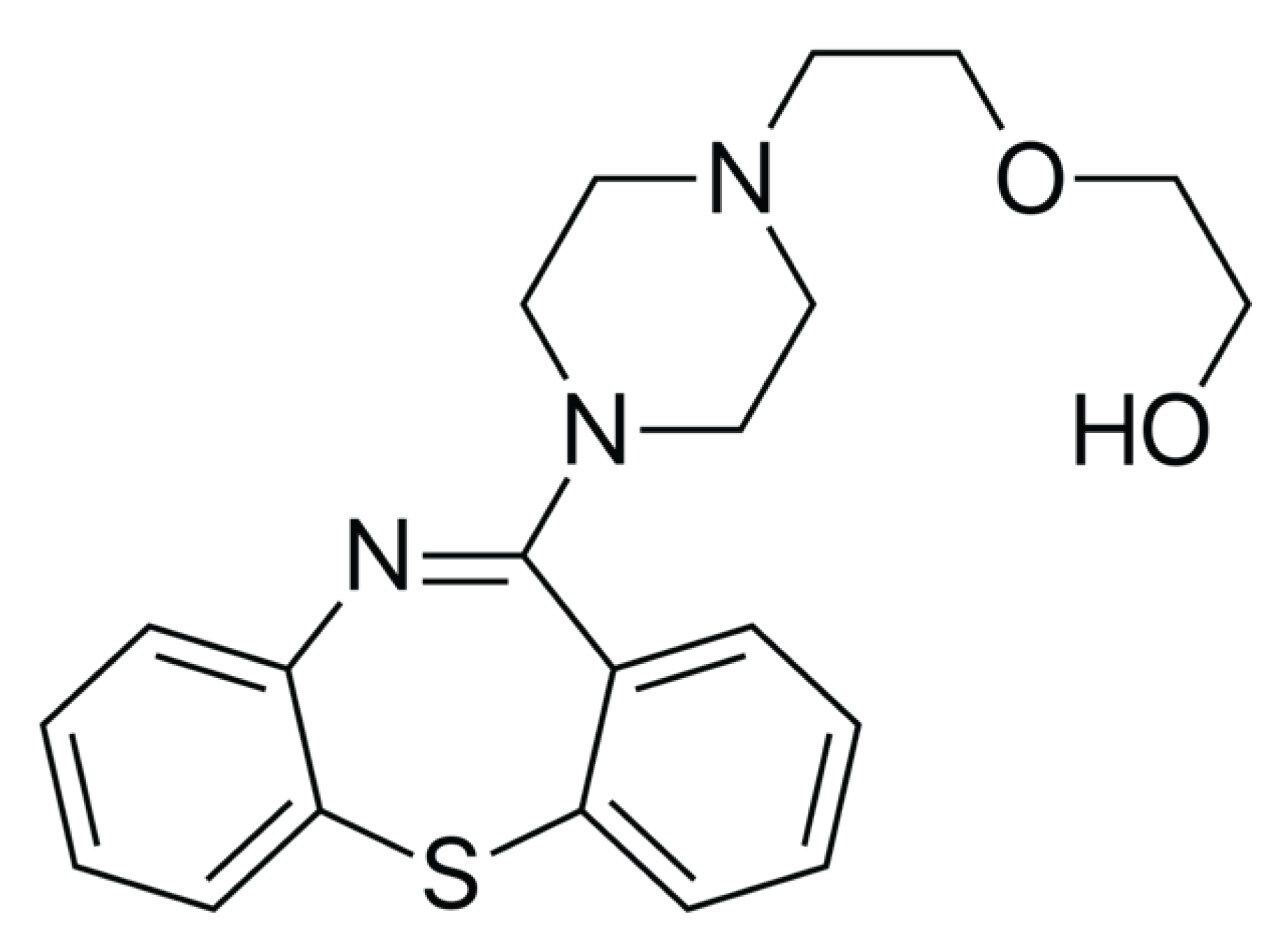
Quetiapine fumarate (QUE) is considered as an atypical or second-generation antipsychotic agent. It is also used for the treatment of depressive episodes associated with bipolar disorder (McEvoy, 2016). QUE was initially developed for the treatment of psychiatric disorders, but due to the added effect of causing sedation, it is now widely used off-label as a treatment for insomnia (Anderson and Vande, 2014) QUE has a chemical structure susceptible to degradation (Figure 2); so, it is important to assess the presence of impurities by suitable analytical techniques such as HRMS. Analytical determination of impurities is often time-constraining and resource-consuming. Analysts require a range of mass spectrometry capabilities as well as sophisticated software to facilitate data processing of these complex impurity data sets. This work demonstrates a systematic workflow that is capable of highly specific and sensitive detection of impurities that are present in quetiapine fumarate (API) drug substance. This workflow-based approach improves the confidence in impurity identification and rapid structural elucidation facilitated by intelligent and user-friendly software. The software performs fragment analysis, correlating the precursor ion information of the low-energy data to that of the fragment ion information of the high-energy data. HPLC with UV detection is a widely used analytical technique for the impurity profiling. Due to its limited sensitivity and specificity, however, HRMS is often required to overcome these issues.
The ACQUITY RDa Detector with SmartMS capabilities enables scientists, who are non-expert users of HRMS, to access accurate mass measurements with in-depth analytical information. Additionally, the waters_connect Software platform acquires, processes and reports results in a complaint-ready framework using a dedicated end-to-end workflow. The full scan with fragmentation function, simultaneously acquires both low and high energy spectra, generating fragment ion information for increased confidence in compound identification.
Experimental
The ACQUITY RDa Detector, coupled to an ACQUITY UPLC I-Class PLUS System, was used for the impurity analysis of Quetiapine fumarate using XBridge C18 (4.6 mm x 150 mm, 3.5 µm) as the stationary phase. The ACQUITY RDa Detector is a compact, benchtop Tof mass spectrometer that has a mass resolution of >10,000 FWHM for routine accurate mass measurements. The system acquired both full scan data and full scan with fragmentation data (data independent acquisition). The UNIFI Application utilizes accurate mass, retention time, and compound fragmentation data to search a customizable application specific library to identify the compound. Figure 1 shows the ACQUITY RDa Detector.
Sample Description
5 mg/mL sample solution of Quetiapine fumarate was prepared using solution A (Buffer: ACN-3:1) as a diluent.
LC Conditions
|
LC system: |
ACQUITY UPLC I-Class PLUS |
|
Detection: |
TUV@250 nm |
|
Vials: |
Total Recovery Vials |
|
Column(s): |
XBridge C18, 4.6 x 150 mm, 3.5 µm |
|
Column temperature: |
45 °C |
|
Sample temperature: |
10 °C |
|
Injection volume: |
20 µL |
|
Flow rate: |
1.5 mL/min |
|
Buffer: |
6.2 grams of Ammonium acetate in 2 L Water and adjust the pH 9.2 with Ammonium hydroxide |
|
Mobile phase A: |
1500 mL of Buffer + 500 mL of Acetonitrile |
|
Mobile phase B: |
Acetonitrile |
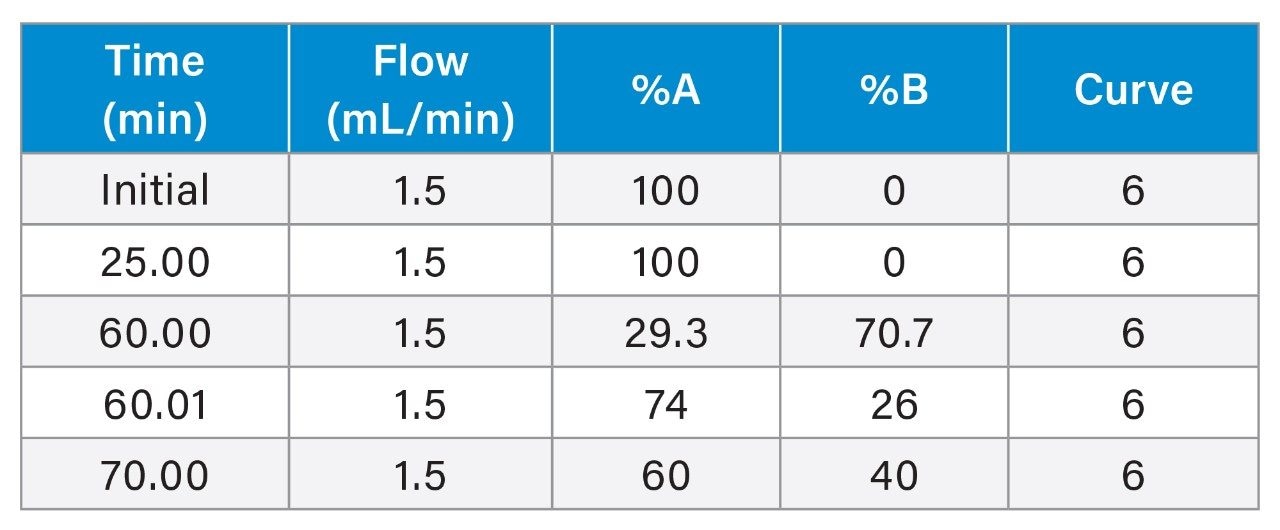
Gradient Table
MS Conditions
|
MS system: |
ACQUITY RDa Detector |
|
Ionization mode: |
Full scan with fragmentation (pseudo - MSE acquisition) |
|
Acquisition range: |
50–2000 m/z |
|
Capillary voltage: |
1.5 kV |
|
Fragmentation cone voltage: |
50–90 V |
|
Cone voltage: |
30 V |
|
Polarity: |
Positive ion mode |
|
Scan rate: |
5 Hz |
|
Desolvation temperature: |
550 °C |
Data Management
|
Informatics: |
waters_connect v1.9.12 |
Results and Discussion
The ACQUITY RDa Detector utilizes the accurate mass workflows for exact mass measurement required for identification and smart decision making. This work demonstrates the UNIFI Software application workflow for impurity analysis, which can also be extended to degradation studies, as shown in Figure 3.
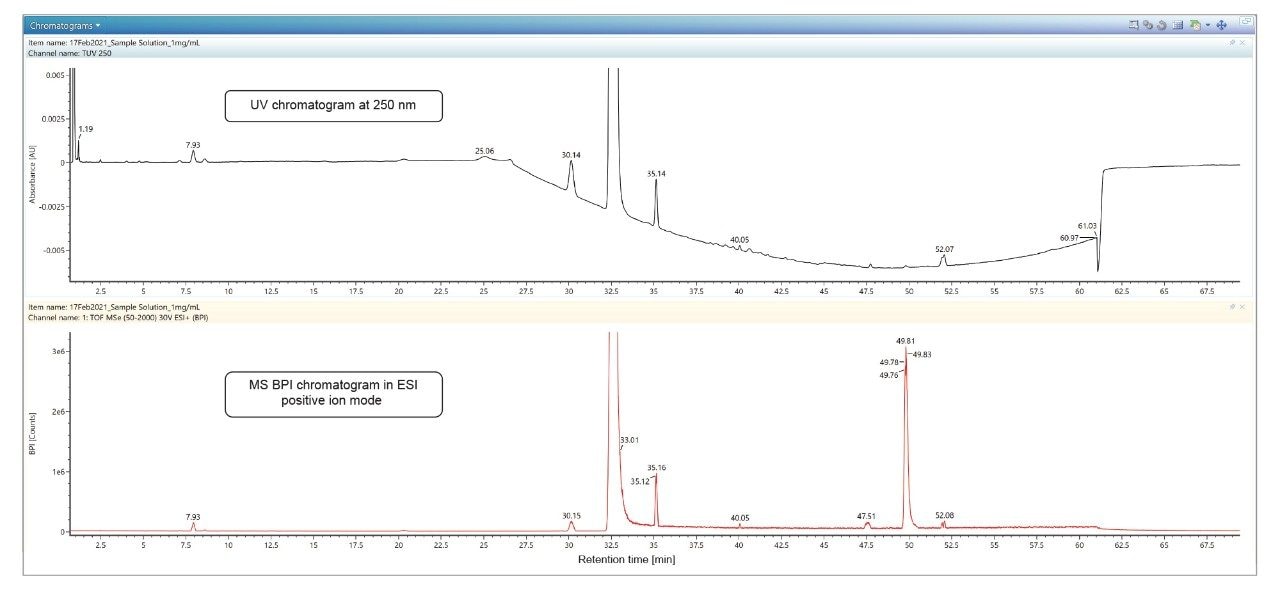
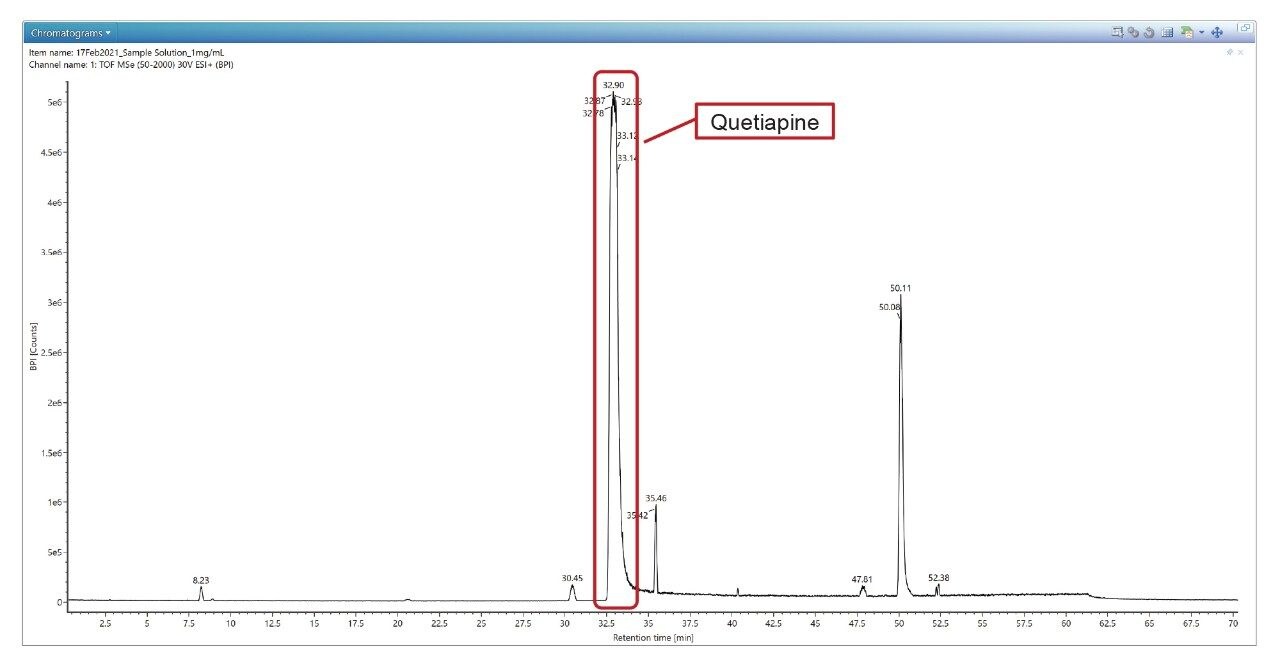
Figure 4 shows a comparison between UV chromatogram and MS BPI chromatogram in ESI positive ion mode.
Quetiapine fumarate API sample (1 mg/mL) was acquired by full scan with fragmentation function of the ACQUITY RDa Detector, where the low energy channel contains the parent ion information and the high energy channel contains the fragment ion information (Figure 5).
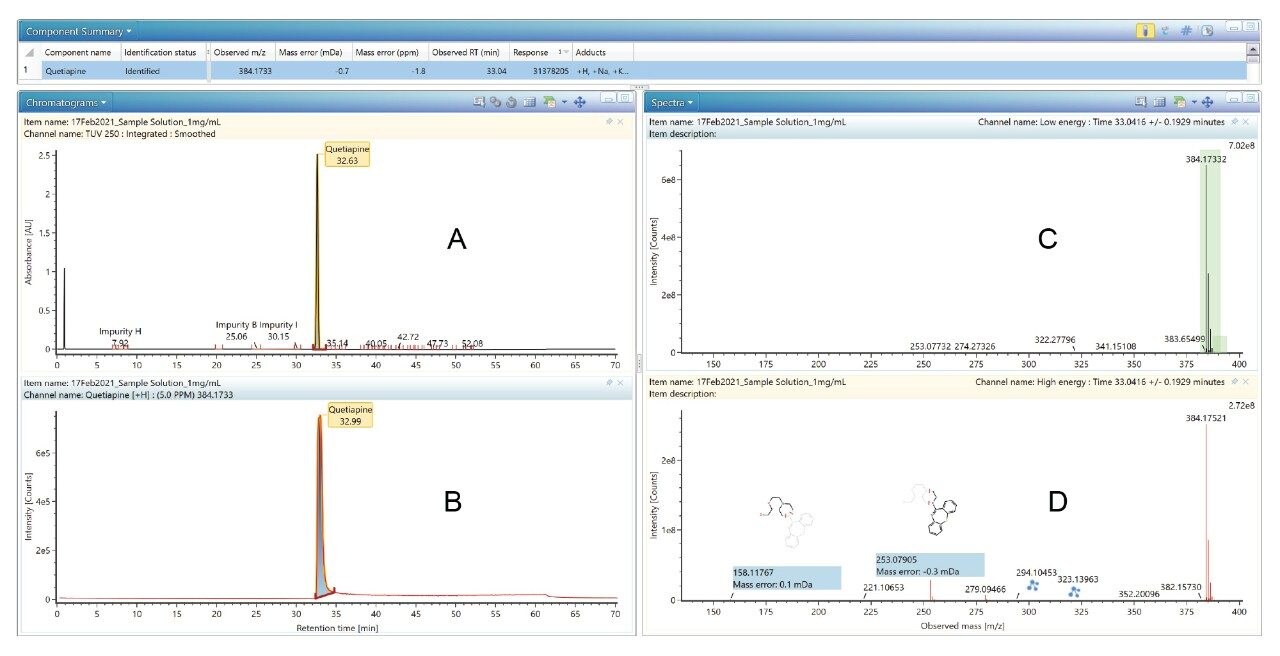
To get an elemental composition for every peak found in a chromatogram, the analyst would typically have to combine MS scans and perform background subtraction for each peak of interest and then generate reports for individual elemental composition. The UNIFI Software populates all impurity peaks integrated in the Tof-MS ES+ chromatographic trace with associated elemental compositions, mass accuracy, and isotope pattern scoring using i-FIT, and displays the results in a single window. Evaluation of the unknown impurity peaks by exact mass and elemental composition using UNIFI Software indicates that the mass accuracy of the API Quetiapine is sub 2 ppm. Nine known impurities were observed with an average mass accuracy of 2 ppm. Software aligns the high and low collision energy data that were simultaneously collected during the pseudo MSE acquisition. The resulting information was displayed in a collective window where the precursor and fragment ions were evaluated spectrally and presented chromatographically.
Here in this application note we have demonstrated two different approaches; one using “Discovery Tool” and the other with “Transformations Tool” to identify an unknown impurity, as shown in Figure 6.

The integrated Discovery Tool allows the user to interrogate unidentified peaks and quickly perform a structural database ChemSpider search for putative identifications of the unknown compound.
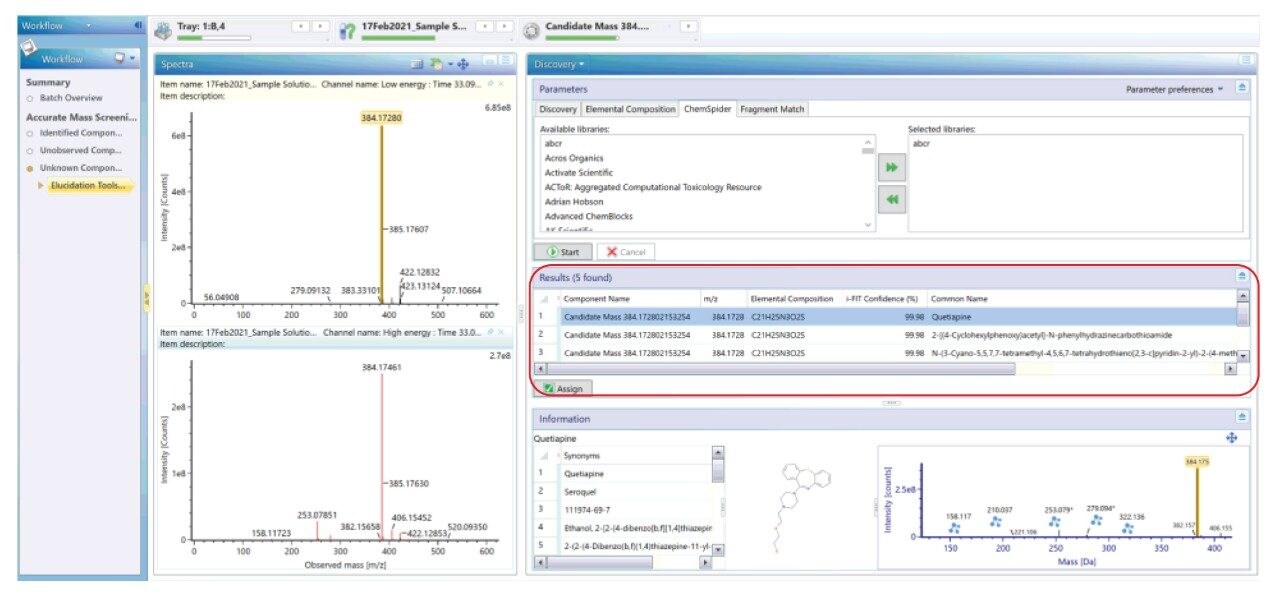
By using Discovery Tool, we can identify the Quetiapine peak at RT of 32.9 minutes as shown in figure 7.
The Discovery Tool takes the accurate mass measured at 32.9 minutes, proposes the elemental formulae and searches in the selected ChemSpider libraries for the possible compounds. In this case, six possible database matches were found. The Discovery Tool also performs an in-silico fragmentation to yield theoretical fragment ions for the database match and compares this spectrum with the acquired fragment ion spectrum. The first candidate found is Quetiapine which matches the spectral composition of the unknown peak as shown in Figure 8.
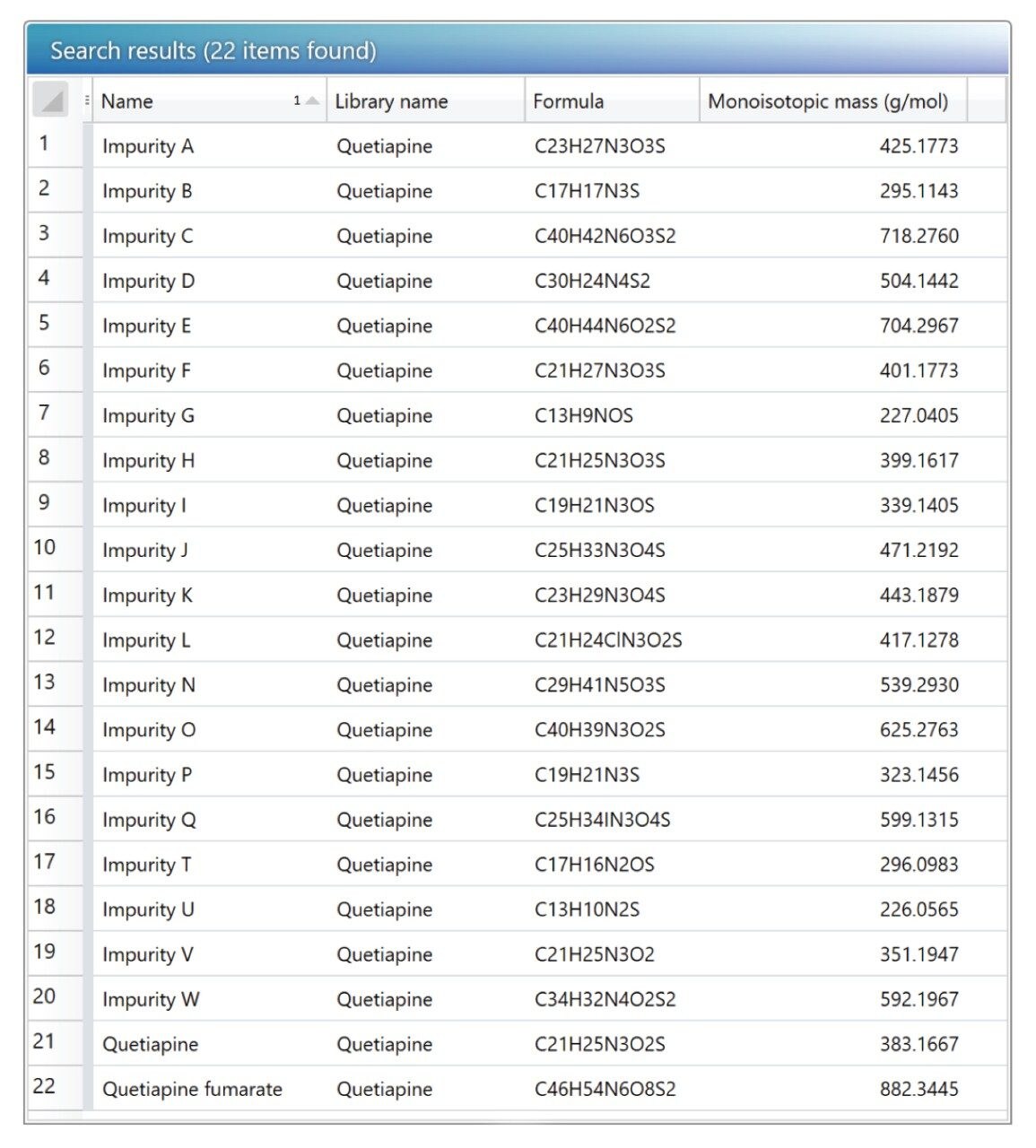
For impurity profiling, the Discovery Tool can be used for API identification and a scientific library created with the identified compounds for routine analysis. Scientific library for Quetiapine and its related impurities has been created by using the available literature as shown in Figure 9.
By using Discovery Tool, we can identify the Quetiapine related impurity peak at RT of 30.45 minutes as shown in Figure 10.
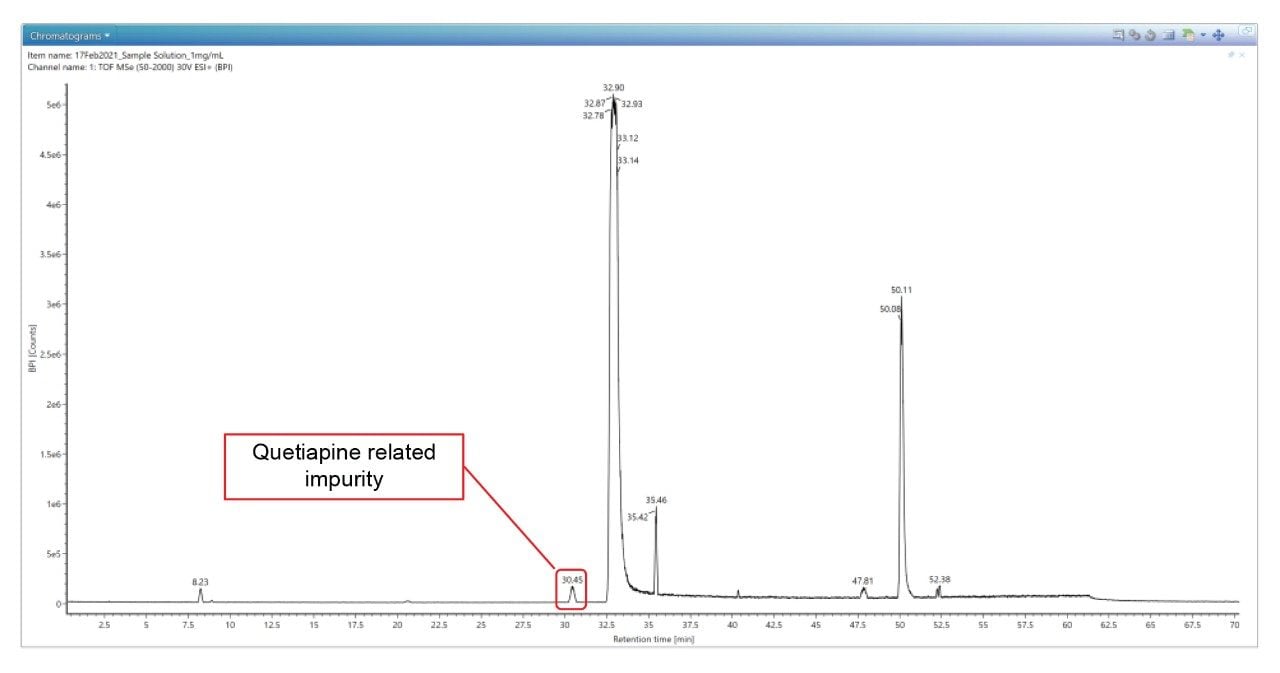
The Discovery Tool takes the accurate mass measured at 30.45 minutes, proposes the elemental formulae and searches in the selected Scientific library for the possible compounds. The Discovery Tool also performs an in-silico fragmentation to yield theoretical fragment ions for the database match and compares this spectrum with the acquired fragment ion spectrum. The candidate found is Impurity-I which matches the spectral composition of the unknown peak as shown in Figure 11.
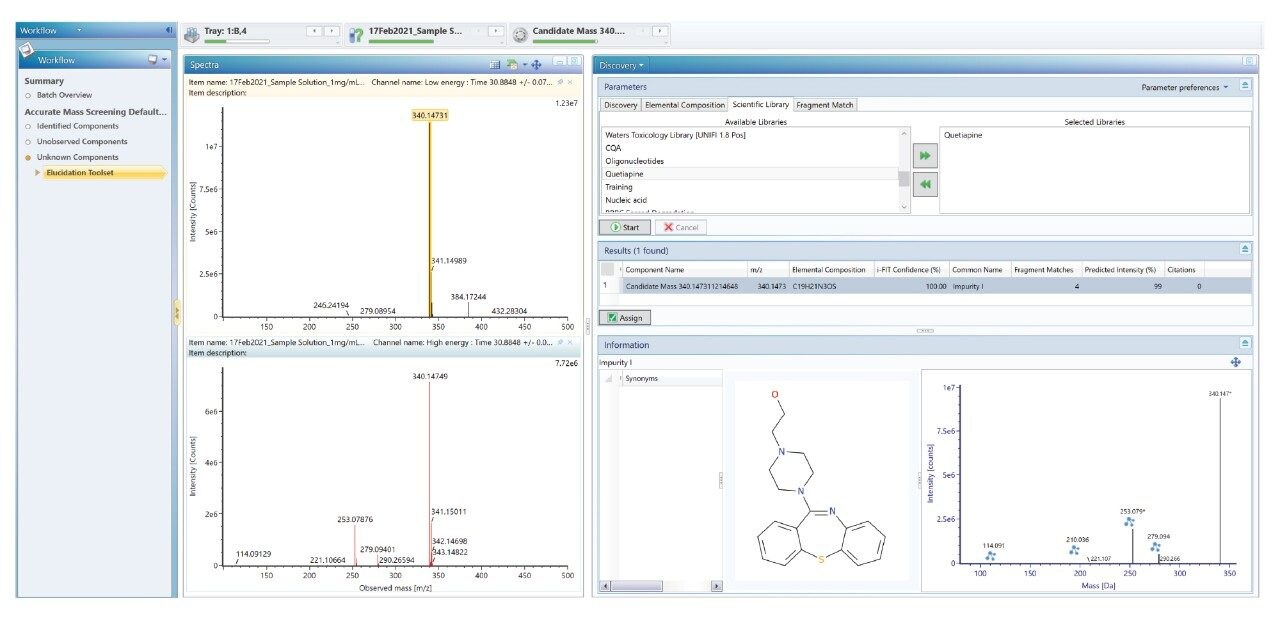
Figure 12 demonstrates the identification of Quetiapine and its related impurities by using the scientific library.

In the initial stage, UNIFI’s Discovery Tool can be used to find the possible identity of the API and impurities. Alternatively, impurities related to API which are potential transformations (oxidations, reductions, dealkylations, etc.) were then successfully identified using the inbuilt transformation tool within the UNIFI application. The suspected impurities are shown within the component summary where all peaks are listed that match the m/z of predicted impurities (Figure 13). The chromatogram and spectral view are also displayed, with the automatic fragment ion assignment in the high energy spectrum annotated with the blue icon (Figure 13). Incorporating the impurity analysis workflow, shown on the left-hand side of Figure 13, is an efficient and effective approach to identifying unknown impurities that are related to API.
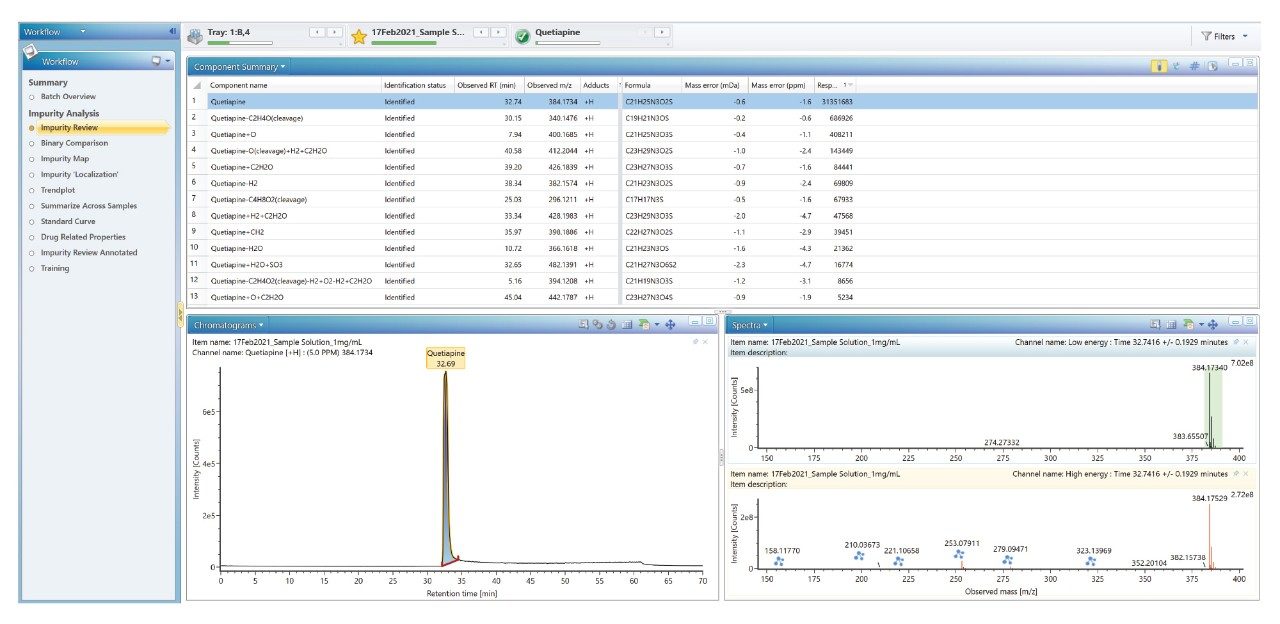
All related impurities of Quetiapine fumarate were identified using ACQUITY RDa Detector with waters_connect platform.
Conclusion
Data collection using the ACQUITY RDa Detector coupled with the ACQUITY UPLC I-Class PLUS System and TUV provided ample sensitivity, and superior mass accuracy to identify many of the impurities in the quetiapine fumarate drug substance. Pseudo MSE provided simultaneous acquisition of both high and low energy data, maximizing the information gathered from a single injection. This analytical workflow was followed by a data processing workflow that streamlined the fragment analysis and structural elucidation process and provided greater confidence in the end results.
The identification of quetiapine and its 9 impurities were rapidly confirmed. The combination of the software tools, along with the optimized instrument configurations for impurity analysis and efficient pseudo MSE acquisition, provided a systematic workflow approach that can readily be applied to identify and confirm known and unknown peaks in impurity profiling. This workflow-based approach delivers the rapid and systematic set of comprehensive results that are needed to identify and confirm impurities in an API impurity profiling study.
References
- Anderson, S., and Vande, J. (2014). Quetiapine for Insomnia: A Review of the Literature. American Journal of Health‐System Pharmacy, 71, 394–402. https://doi.org/10.2146/ajhp130221.
- Bharathi, C., Prabahar, K., Prasad, C., Srinivasa, R., Trinadhachary, G., Handa, V., Naidu, A. (2008). Identification, Isolation, Synthesis and Characterization of Impurities of Quetiapine Fumarate. Pharmazie, 63, 14–19.
- Fisher, D., Handley, S., Flanagan, R., and Taylor, D. (2012). Plasma Concentrations of Quetiapine, N‐desalkylquetiapine, O‐desalkylquetiapine, 7‐ hydroxyquetiapine, and Quetiapine Sulfoxide in Relation to Quetiapine Dose, Formulation, and other factors. Therapeutic Drug Monitoring, 34, 415–421. https://doi.org/10.1097/FTD.0b013e3182603f62.
- Jones, MD, et al. Identification and Characterization of an Isolated Impurity Fraction: Analysis of an Unknown Degradant Found in Quetiapine Fumarate. Waters Corporation. 2009; 720003079EN.
- Impurity Isolation and Scale-up from UPLC: Analysis of an Unknown Degradant Found in Quetiapine Fumarate. Waters Corporation. 2009; 720003078EN.
720007354, September 2021