Separation and Detection of an Azido Impurity in Sartan Drug Substances Using the XSelect CSH Phenyl-Hexyl Column by UHPLC-UV-MS
Abstract
Recent genotoxic impurity concerns around “sartan” pharmaceuticals, which are angiotensin II receptor blockers (ARBs), have prompted recall notices from several regulatory agencies. Low safety threshold levels for these impurities necessitate a strong need for appropriate analytical methods. However, current United States Pharmacopeia (USP) monographs may not provide adequate separation of the impurity from the active pharmaceutical ingredients (APIs). Here, a new method is compared against a USP monograph and demonstrates the potential of an alternative column chemistry, the XSelect CSH Phenyl-Hexyl, in separating a mixture of sartans (irbesartan, losartan, valsartan) and an azido impurity (5-[4’-(azidomethyl)-[1,1’-biphenyl]-2-yl)-1H-tetrazole). Improved selectivity and resolution were achieved for all compounds using the ACQUITY Arc System coupled to a PDA and an ACQUITY QDa Mass Detector, allowing a rapid 3-minute isocratic method to be developed with good MS linearity for each compound.
Benefits
- Improved resolution of sartan drugs and an azido impurity using the XSelect CSH Phenyl-Hexyl Column
- Accurate analysis of an azido impurity in sartan drug substances using LC-UV-MS
- Fast and reproducible quantification from 16 ng/mL–50 µg/mL of the azido impurity using a 3-minute isocratic method
Introduction
Irbesartan, losartan, and valsartan are angiotensin II receptor blockers (ARBs) that are used to treat high blood pressure and diabetic nephropathy (kidney disease).1 A recall of these “sartan” drugs was issued in June 2021 due to the suspected presence of the genotoxic azido impurity, 5-[4’-(azidomethyl)-[1,1’-biphenyl]-2-yl)-1H-tetrazole.2 This azido impurity is an intermediate in the synthesis pathway for each of the active pharmaceutical ingredients (APIs) and comprised of a similar backbone structure. As shown in Figure 1, all four compounds contain a tetrazole ring bonded to a phenyl group, which incorporates another phenyl group at its ortho-position. The differences between the compounds is at the para-position of the second phenyl ring and require suitable analytical methods for accurately detecting and quantifying the azido impurity and the sartan APIs. This is made more challenging by the similar chemical structures.
In this work, we present a UHPLC method with dual UV-MS detection for the quick and accurate, 3-minute analysis of this azido impurity, 5-[4’-(azidomethyl)-[1,1’-biphenyl]-2-yl)-1H-tetrazole, and the sartan APIs irbesartan, lorsartan, and valsartan using an XSelect CSH Phenyl-Hexyl Column. An ACQUITY Arc System with PDA detection coupled to an ACQUITY QDa Mass Detector was used. This fast and simple method employs isocratic conditions with MS-friendly mobile phases for full separation of the four compounds. This combination of UV and MS detection allowed accurate determination and mass confirmation of the azido impurity and APIs with improved assay sensitivity, achieving a lower limit of detection of 16 ng/mL for the azido impurity, using a 1 µL injection.
Experimental
Sample Description
Four analyte stock solutions were prepared at 1 mg/mL using 50:50 acetonitrile:water as the sample diluent. All subsequent dilutions used 50:50 acetonitrile:water as the diluent. Sample concentrations vary throughout testing.
LC Conditions
|
LC systems: |
ACQUITY Arc with a 2998 PDA Detector and ACQUITY Qda Mass Detector |
|
Detection: |
UV @ 230 nm |
|
SIRs of analytes (Figure 1) |
|
|
Vials: |
LCMS Certified Clear Glass Vial 2 mL (p/n: 600000751CV) |
|
Column(s): |
XSelect BEH C18, 3.0 x 50 mm, 2.5 µm (p/n: 186006033) |
|
XSelect CSH Phenyl-Hexyl, 3.0 x 50 mm, 2.5 µm (p/n: 186006129) |
|
|
Column temp.: |
30 °C |
|
Sample temp.: |
10 °C |
|
Injection volume: |
1 µL |
|
Flow rate: |
1.7 mL/min–scaled from USP monograph |
|
0.85 mL/min–method development flow rate |
|
|
Mobile phase A: |
Water |
|
Mobile phase B: |
Acetonitrile |
|
Mobile phase C: |
50:50:0.1 (Acetonitrile:water:acetic acid) USP monograph mobile phases |
|
Mobile phase D: |
2% Formic acid in water |
MS Conditions
|
MS system: |
ACQUITY QDa |
|
Ionization mode: |
SIRs in ESI+ |
|
Acquisition range: |
See Figure 1 |
|
Capillary voltage: |
1.5 kV |
|
Cone voltage: |
15 V |

Data Management
|
Chromatography software: |
Empower 3 Feature Release 4 |
Results and Discussion
Preliminary method development efforts for the compounds in Figure 1 were focused on creating one generic method, which could separate, detect, and quantify the sartans and azido impurity. For these reasons, analysis was performed using the ACQUITY Arc System with PDA Detector, integrated with an ACQUITY QDa Mass Detector for mass confirmation. As a starting point, the USP monograph for valsartan was investigated, as both the irbesartan and losartan monographs use phosphoric acid and phosphate buffer in their mobile phases, which are not compatible with MS detection. The USP monograph for valsartan employs a combination of acetonitrile:water:acetic acid as the mobile phase, making it the only sartan monograph compatible for MS detection.
For analysis, stock solutions of the four compounds were prepared as described in the USP monograph.3 Individual standard injections at 0.2 mg/mL (APIs) and 0.02 mg/mL (azido impurity), as well as a mixture of the four compounds at the indicated concentrations, were performed using an XBridge BEH C18 3.0 x 50 mm 2.5 µm, which is scaled from the USP monograph. Full LC and MS details are listed in the experimental section. Figure 2 shows the UV chromatographic results obtained using this method.
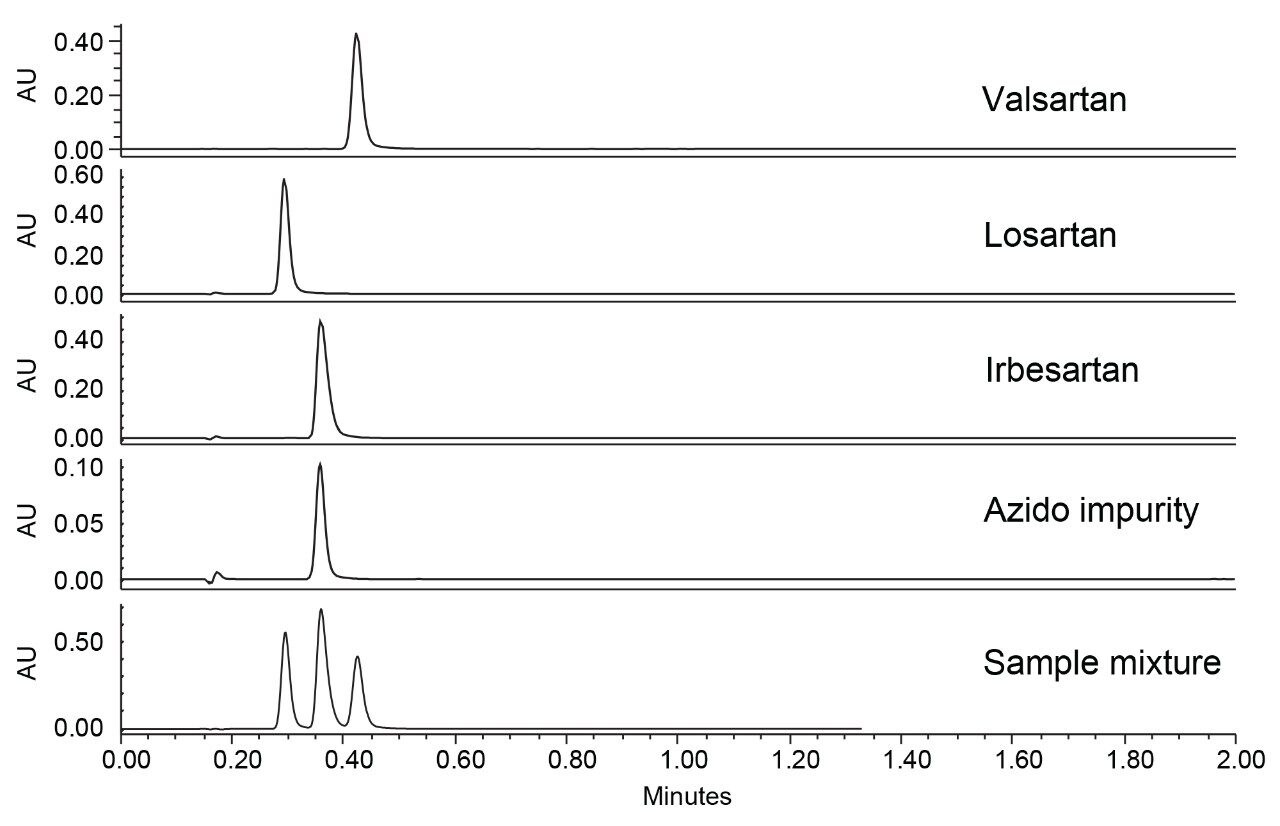
Inadequate separation of the three sartans and the azido impurity using the scaled USP monograph and XSelect XBridge BEH C18 Column can be seen in Figure 2, where the azido impurity co-elutes with the irbesartan peak. From this result, a generic method for full separation of the impurity and sartans could not be achieved indicating further method development was needed. Given the closely related, multi-ringed structures of the four compounds, we chose to test the XSelect CSH Phenyl-Hexyl Column next. This column employs both a different base particle and a different bonded ligand compared to the XBridge BEH C18 Column. The CSH particle is similar to the BEH in that both are hybrid particles; however, the former maintains a slight positive charge on the particle which is not present on the BEH particle. This slight charge acts in two ways. First, the slight positive charge improves peak shape for basic analytes at low pH due to weak ionic repulsion. Second, the positive charge on the particle can act as a weak anion exchanger under the right circumstances, leading to differing selectivity for acidic probes. The phenyl-hexyl bonded ligand differs from the C18 ligand in that secondary pi-pi interactions can occur between the ligand and analytes, which could prove advantageous when analyzing compounds with multiple phenyl rings, like the sartans.
By using this XSelect CSH Phenyl-Hexyl Column, we could achieve better selectivity and improved resolution for the separation of the sartan drugs and azido impurity. Moreover, this allowed us to optimize the method and greatly reduce the run time to a 3-minute isocratic method, which is shown in Figure 3. In addition, the use of formic acid as a mobile phase modifier was found to be adequate to achieve the desired separation.
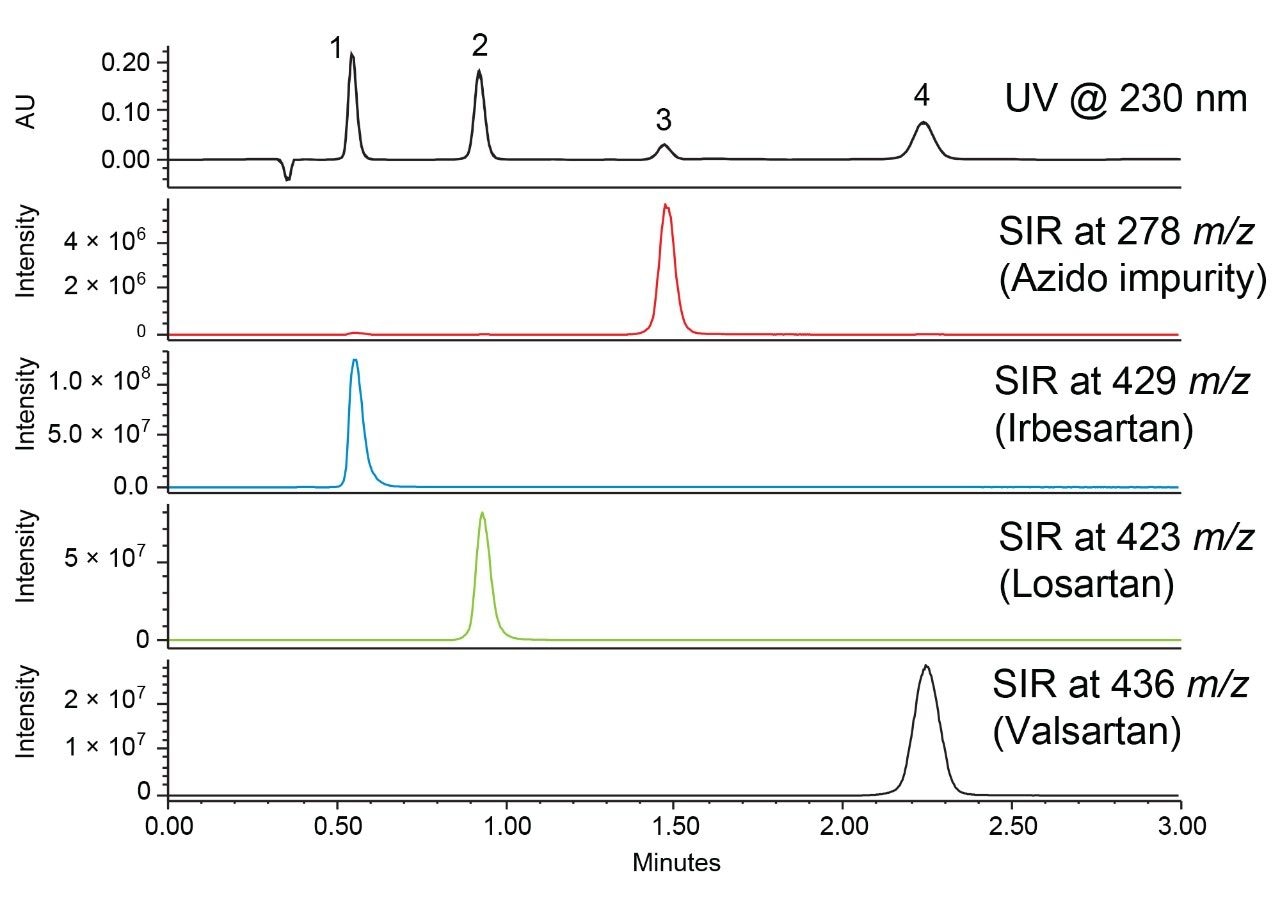
Figure 3. UV and SIR chromatograms of the azido impurity, irbesartan, losartan, and valsartan obtained using an XSelect CSH Phenyl-Hexyl, 3.0 x 50 mm, 2.5 µm Column. Isocratic separations were performed with an ACQUITY Arc System coupled to a PDA detector and ACQUITY QDa for mass detection using a flow rate of 0.85 mL/min, a column temperature of 30 °C, and formic acid modified mobile phase (40:60 acetonitrile:water).
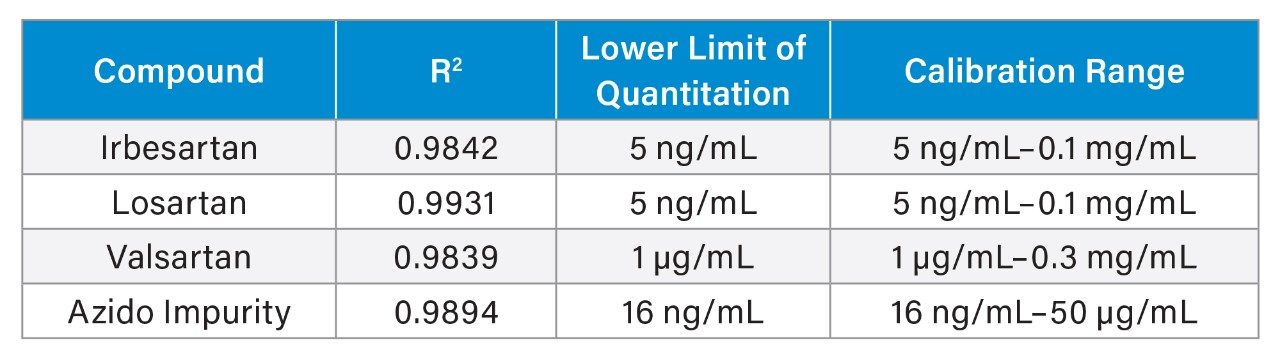
Following LC method optimization, MS sensitivity and linearity were evaluated for the sartan drugs and azido impurity using the ACQUITY QDa Mass Detector. Calibration curves (n=3) were generated for all four compounds MS quantitative performance including lower limits of quantitation, linear regression (R2), and calibration curve ranges are highlighted in Table 1. To confirm MS accuracy of the azido impurity, a 50 ng/mL sample was assessed for recovery/accuracy and repeatability. The average % recovery (n=9) was determined to be 97.4%, with %RSDs of 4.2%, easily meeting recommended criteria of 85-115% for recovery and RSDs.
Conclusion
A fast, generic LC-UV-MS method was successfully developed for the accurate and reproducible analysis of the azido impurity(5-[4’-(azidomethyl)-[1,1’-binyphel]-2-yl)-1h-tetrazole) and sartan APIs. The XSelect CSH Phenyl-Hexyl Column provided excellent selectivity versus historically used C18 Columns, effectively separating losartan, valsartan, irbesartan, and the azido impurity. An ACQUITY Arc System with PDA detection coupled to a ACQUITY QDa Mass Detector was used for quick and accurate peak identity confirmation. Overall, this proof-of-concept method delivers a simple, under 3-minute solution for the determination of the azido impurity, 5-[4’-(azidomethyl)-[1,1’-biphenyl]-2-yl)-1H-tetrazole, from sartan APIs.
References
- Angiotensin II Receptor Blockers. Mayo Clinic Website. Accessed 30-Aug-2021. https://www.mayoclinic.org/diseases-conditions/high-blood-pressure/in-depth/angiotensin-ii-receptor-blockers/art-20045009.
- Government of Canada Recalls and Safety Alerts. Multiple Lots of Irbesartan, Losartan, and Valsartan Drugs Recalled Due to Azido Impurity. Accessed 16-Aug-2021. https://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2021/75715a-eng.php.
- USP Monograph Conditions Valsartan Tablets. USP43-NF38-4579, USP42-NF37-4539, USP41-NF36 2S-8984. Accessed via USP-NF mobile app 19-July-2021.
720007369, Revised September 2021