
In a global economy, there is often a challenge to implement and maintain a competitive advantage. New instrumentation is desired as it may provide improvements in both performance and usability. To integrate new instrumentation, it is important to harmonize the approach across many sites and to control for method and other variables from one laboratory to another. To address these challenges, it is advisable to use a risk-based approach and control strategy. In this application note, a global interlaboratory method transfer study of a USP impurities method was conducted at eight participating sites. Preliminary testing was conducted at the sending laboratory site, providing key information for the control strategy and transfer process. With this process in place, the system and method were demonstrated to meet system suitability requirements routinely.
In a global economy there is often a challenge to implement and maintain a competitive advantage. New instrumentation is desired as it may provide improvements in both performance and usability. When assessing new instrumentation, particularly for method transfer, it is important to control and understand system configurations. Furthermore, specific method conditions and other variables will need to be controlled to minimize variability from one laboratory to another. To address these challenges, a solid understanding of risks that can impact method performance and control strategies to minimize the risks need to be implemented.
In this application note, a global interlaboratory method transfer of a USP organic impurities method was conducted across eight sites around the world. The transfer was performed on Arc HPLC Systems. The sending laboratory site conducted robustness and verification testing prior to the study. The process was multi-faceted and included a risk assessment at the sending laboratory site. The control strategies were providing a standard operating procedure (SOP) and key materials from the sending laboratory site to the receiving laboratories. With these control strategies, the system and method variables were controlled to allow for successful method transfer.
The method was based on the USP monograph for Quetiapine Fumarate Impurities,1 with no adjustments.
The method requires both a system suitability reference standard (RS) and a quetiapine fumarate RS. The system suitability solution was prepared from the USP quetiapine system suitability RS (USP p/n 1592715) and consists of a mixture of quetiapine, quetiapine desethoxy (1–5%), related compound G and related compound B standard. The system suitability solution was prepared at 1 mg/mL in diluent (86:14 Solution A/Solution B) from the quetiapine system suitablity RS. The standard solution was prepared utilizing the USP quetiapine fumarate RS and was prepared at a concentration of 0.001 mg/mL in diluent.
The drug substance was obtained from Hangzhou Think Chemical Co., Ltd. and past the date of expiration. The sample was prepared at 1.0 mg/mL in Solution A.
|
Column: |
XBridge C8 3.5 µm, 4.6 x 150 mm (Waters p/n: 186003055) |
|
Column temp.: |
45 °C |
|
Sample temp.: |
10 °C |
|
Injection volume: |
20 µL |
|
Detection: |
250 nm |
|
Data rate: |
10 Hz |
|
Flow rate: |
1.5 mL/min |
|
Run time: |
70 minutes |
|
Buffer: |
3.1 g/L Ammonium acetate in water. Add 2 mL of 25% ammonium hydroxide to each 1 L of solution pH = NLT 9.2 |
|
Solution A: |
25:75 ACN:buffer |
|
Solution B: |
Acetonitrile |
|
Needle wash: |
50:50 Water:acetonitrile |
|
Purge solvent: |
50:50 Water:acetonitrile |
|
Seal wash: |
90:10 Water:acetonitrile |
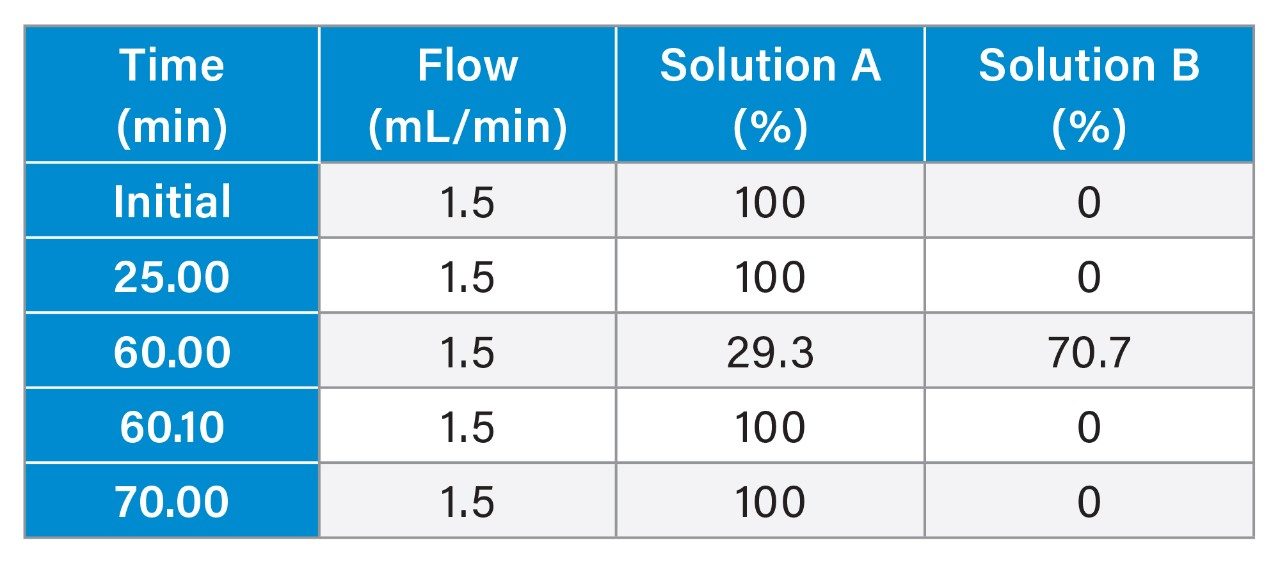
|
Empower 3 Chromatography Data Software |
|
System: |
Arc HPLC System (QSM-R, FTN-R, and CHC) |
|
Detection: |
2998 (PDA) or 2489 (TUV) |
|
Configuration: |
Passive preheater (preferred) |
|
Flow cell: |
Analytical |
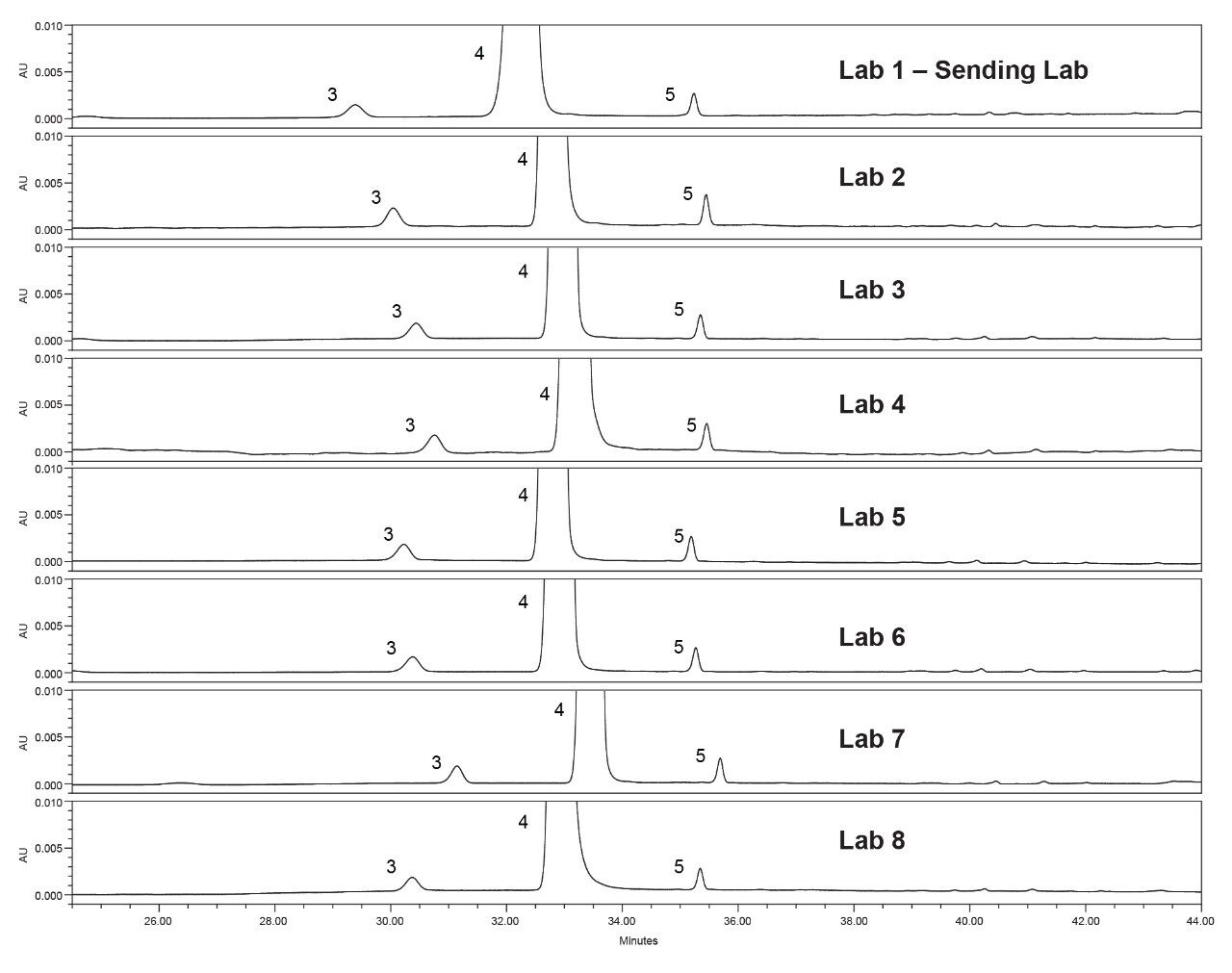
Method transfer of the USP quetiapine fumarate Impurities method1 was conducted across sites around the world. The laboratories were located in Milford, MA, USA (sending laboratory) and 7 receiving labs – Milford, MA 2nd lab, Turkey, India, Singapore, China, France, and North Carolina, USA. The study included verifying the system suitability requirements and quantitative analysis of a drug substance. All analyses were performed on the Arc HPLC System with either a Tunable Wavelength (TUV) 2489 Detector or a photodiode array (PDA) 2998 Detector. Each analysis was assessed using the system suitability requirements, as described in the monograph, as well as analyzing the drug substance and comparing the impurity analysis. The system suitability criteria were based on the resolution of two critical pairs in the system suitability solution, and the tailing, retention time %RSDs and area %RSDs from the standard solution.
To ensure method performance was not impacted by numerous variables, a risk-based approach was performed. This approach consisted of multiple steps:
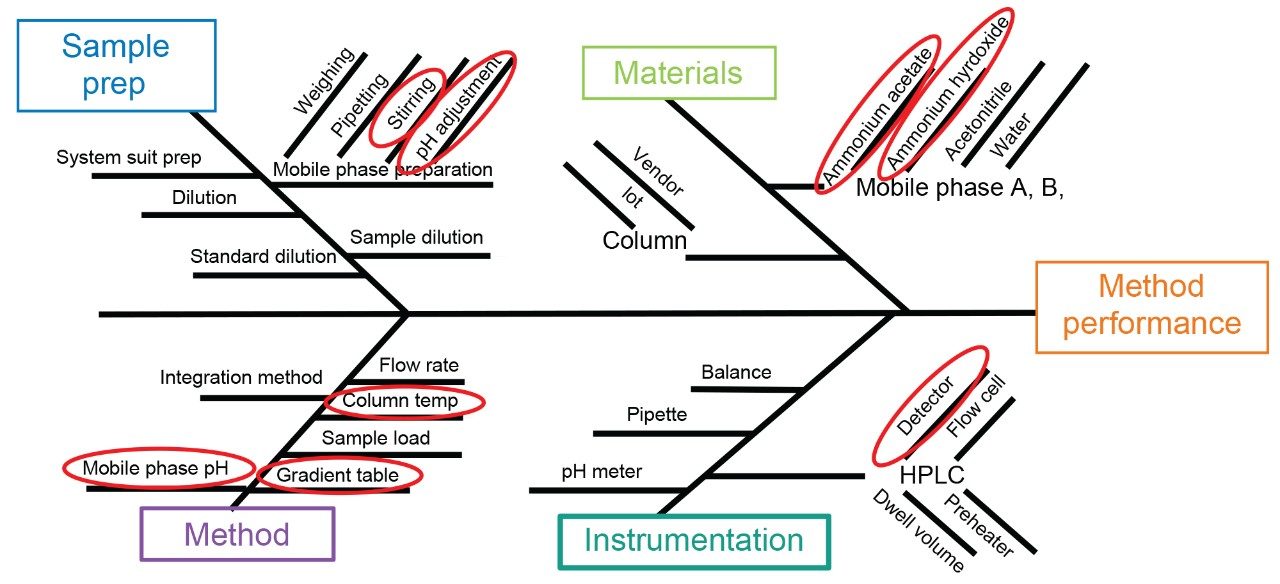
The risk assessment was first performed by reviewing the method and gathering input from subject matter experts and scientists familiar with the method. A fishbone or Ishikawa diagram (Figure 1) was used to identify potential variables that could impact the performance of the method, which includes meeting system suitability and quantitative measurement. The potential causes of method variability were divided into four categories, including sample preparation, the materials, the method parameters and the instrumentation. For each category, specific characteristics were identified as risks that might impact the method performance. Based on gathered knowledge, those that would produce the most variability was identified (red circle). For example, column temperature, was identified as a key variable since the method required an elevated temperature of 45 °C. Materials including ammonium acetate and ammonium hydroxide can change concentration over time, resulting in retention time variability. Other variables identified were stirring and pH adjustment of the mobile phase, mobile phase pH and detector.
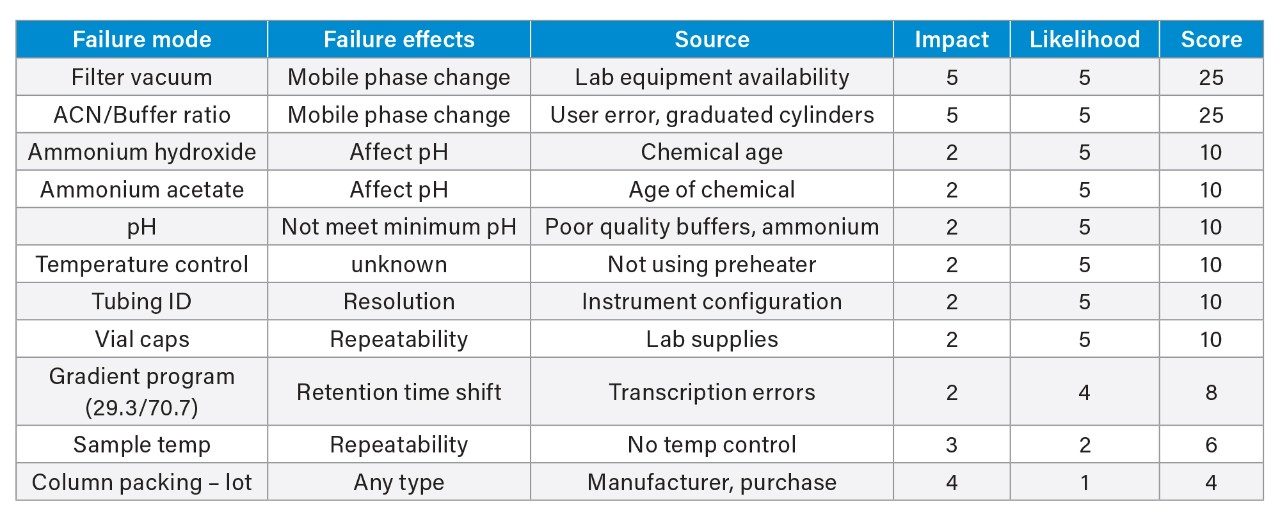
Once the key causes of variability were identified, the scientists ranked or scored the variables for their impact on the method performance. Further risk assessment was performed by identifying failure modes (based on the input above) and their effects on the method performance. Each failure mode was scored on impact and likelihood. Key failure modes, based on previous studies, were identified as the buffer/acetonitrile ratio of solvent A and the pH adjustment of the buffer solution. Previous robustness studies had determined that the buffer/acetonitrile ratio impacted selectivity and resolution. Based on this information, the filter vacuum and the buffer/acetonitrile ratio were given high impact. Excessive vacuum on filtration could lead to evaporation of organic impacting the buffer/organic ratio. Each of these failure modes was likely to occur as filtration was performed in the method and combining two solvents on volume can often be impacted by the graduated cylinder class. Other high risks included the buffer solution pH, and temperature control.
To reduce variability for the method transfer study, control strategies were developed based on the results from the risk assessment. These included providing the critical materials and SOP to the receiving lab from the sending lab. A single chemical kit was sent out to all the receiving laboratories and included the standards, the column, and the drug substance. The standards were purchased from the supplier (USP) in a single purchase and the drug substance was a single lot. However, the columns included two different lots. The SOP was written at the sending laboratory and then sent to a second site in Milford, MA for review, comments, and final approval. To ensure each lab was able to replicate the method - one which was unfamiliar to them - a detailed SOP was written. To ensure the instructions were comprehensive and clear, the second site in Milford acted as a beta site and reviewed and provided comments on the SOP. Within the SOP, specific instructions were also implemented to control lab to lab variability. For example, a preheater was required to reduce the impact of lab-to-lab temperature variability.
Lastly, each lab processed the system suitability analyses with a processing method provided within the SOP. However, all data presented in this publication was processed at the sending laboratory site to reduce variability in results due to different processing method parameters.
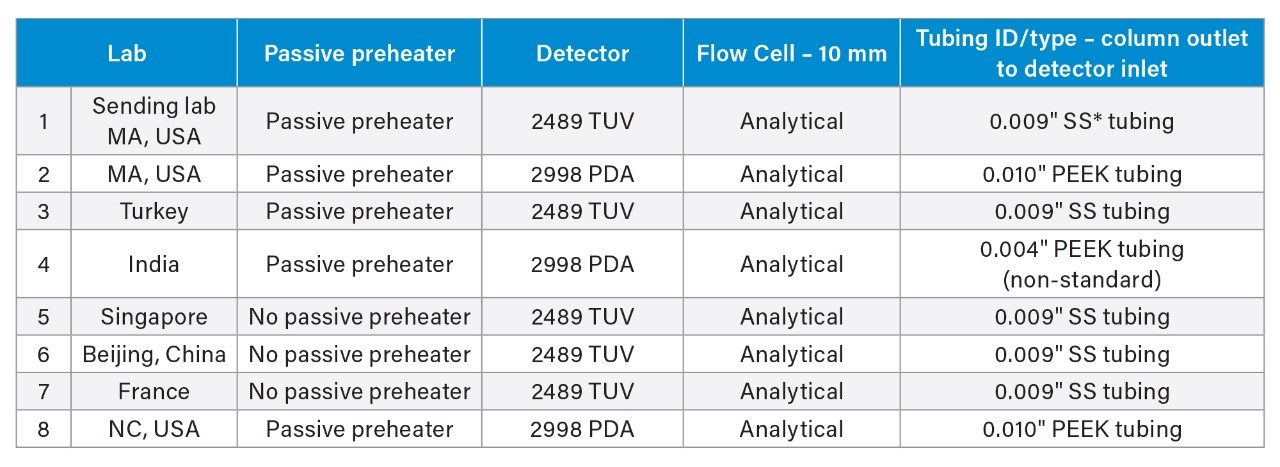
System configuration was also found to impact method performance. To control the system configuration at each site, specific configurations were requested in the provided SOP. However, due to availability of parts, some labs did not have the configuration requested. The receiving laboratories therefore recorded the configuration differences. The important aspects of the system included whether or not a passive preheater was used, the detector (TUV or PDA), the flow cell type, the flow cell path length, and the tubing ID from the outlet of the column to the inlet of the detector. The system configuration from each lab is listed in Table 2.
As described above, the impurities method for quetiapine fumarate consisted of two steps: performing system suitability analysis and quantification of a drug substance. After the system suitability of the system was met, the drug substance was analyzed. Comparison of each standard/sample across the numerous labs was performed.
Based on this information the method transfer was measured based on the following:
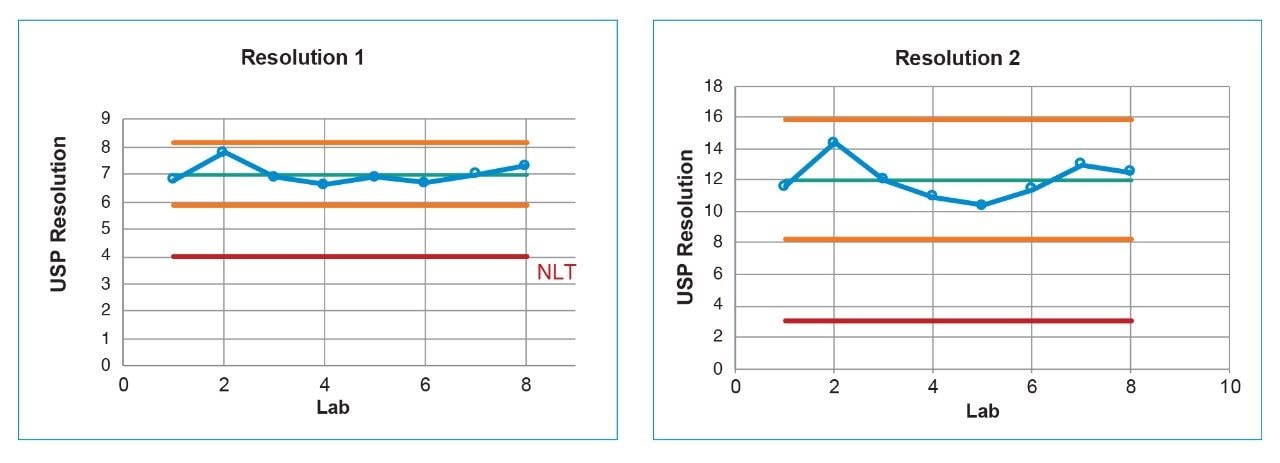
The system suitability solution is used to assess resolution of two critical pairs as defined in the USP method. The first critical pair was quetiapine desethoxy and quetiapine (API) and the second critical pair was related compound G and related compound B. The results for all laboratories can be found in Figure 2. As shown, all the laboratories met the USP resolution criteria for the system suitability solution for both resolution 1, and resolution 2. To ensure the study was in a state of control, the resolution values obtained were analyzed in a control chart. The control chart (Figure 3) shows that the values were all within 3 standard deviations of the mean and that the +/-3 standard deviations also were within the system suitability requirements of the method.
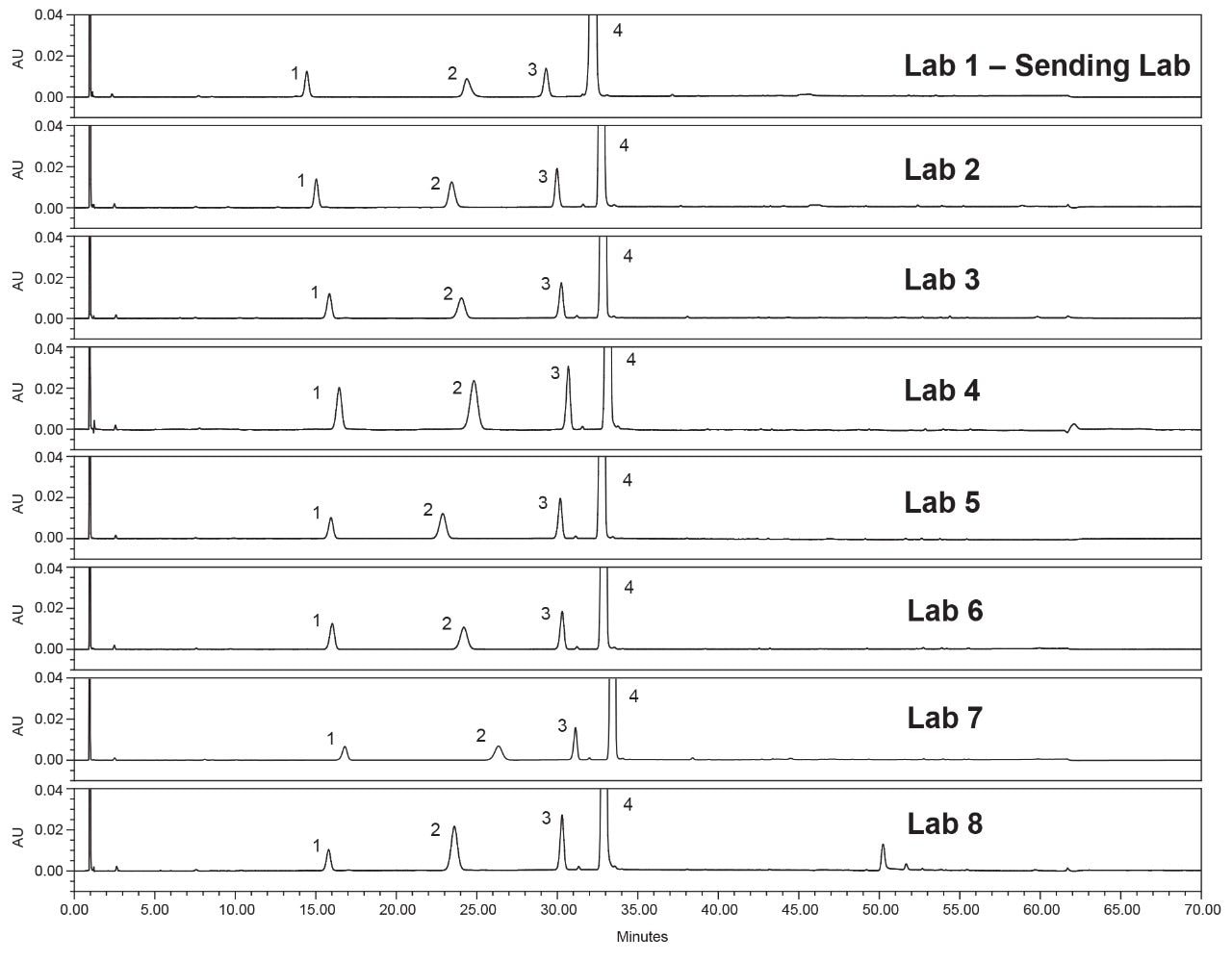
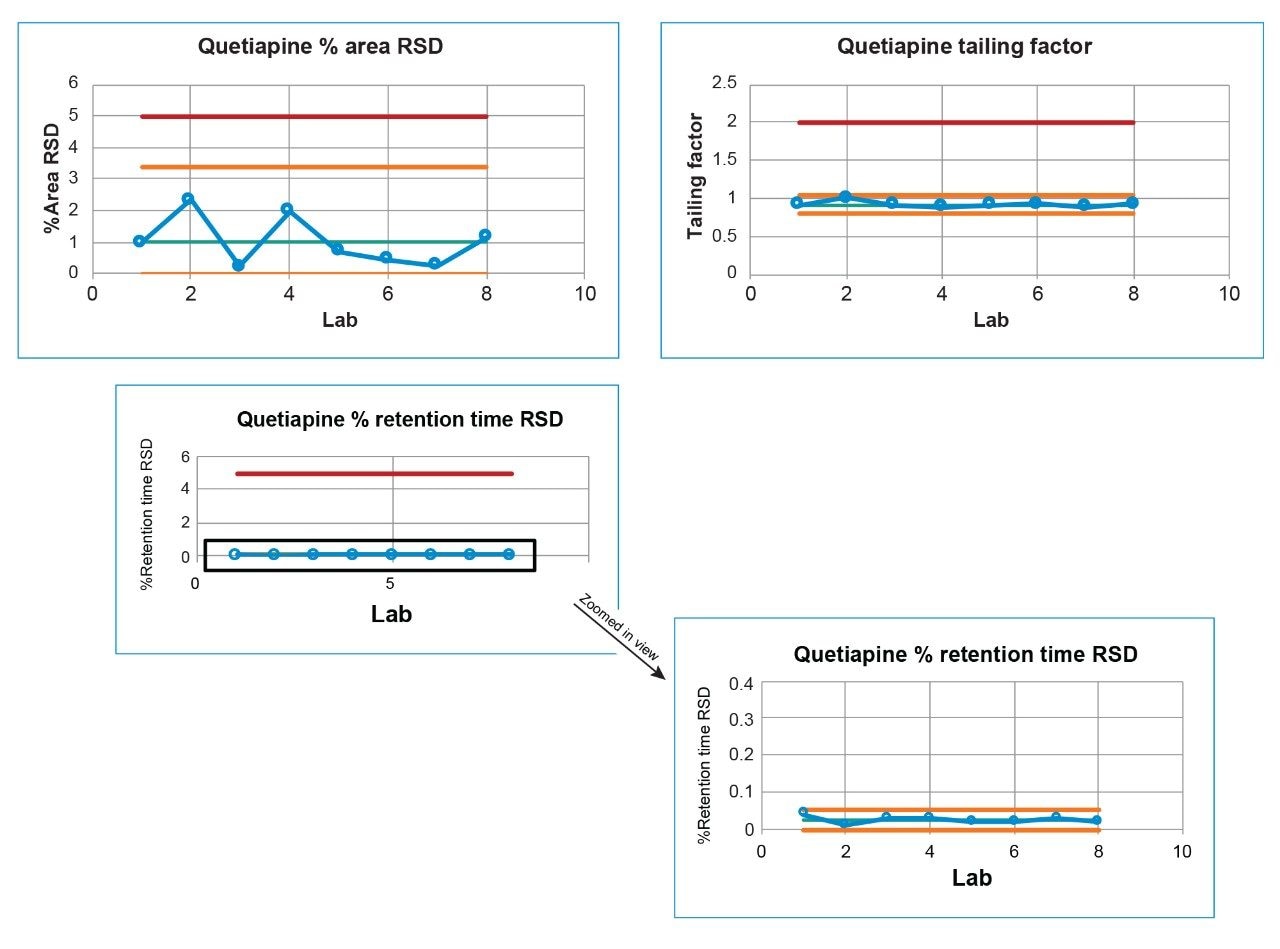
The second part of the system suitability required the analysis of the standard solution for tailing factor, retention time %RSDs, and area %RSDs. The chromatographic results for the standard solution from all the laboratories (Figure 4) show that all the system suitability requirement results were well within the specifications, including the tailing factor of not more than 2.0, the retention time RSD of not more than 5% and the area %RSDs of not more than 5%. When reviewing the results, the area %RSD was greater for some systems while USP tailing and retention time %RSD were comparable (Figure 5). The systems in those labs (2, 4, and 8) consisted of PDA detectors, while all other labs used TUV detectors. The detector was determined to be a key reason for the difference.
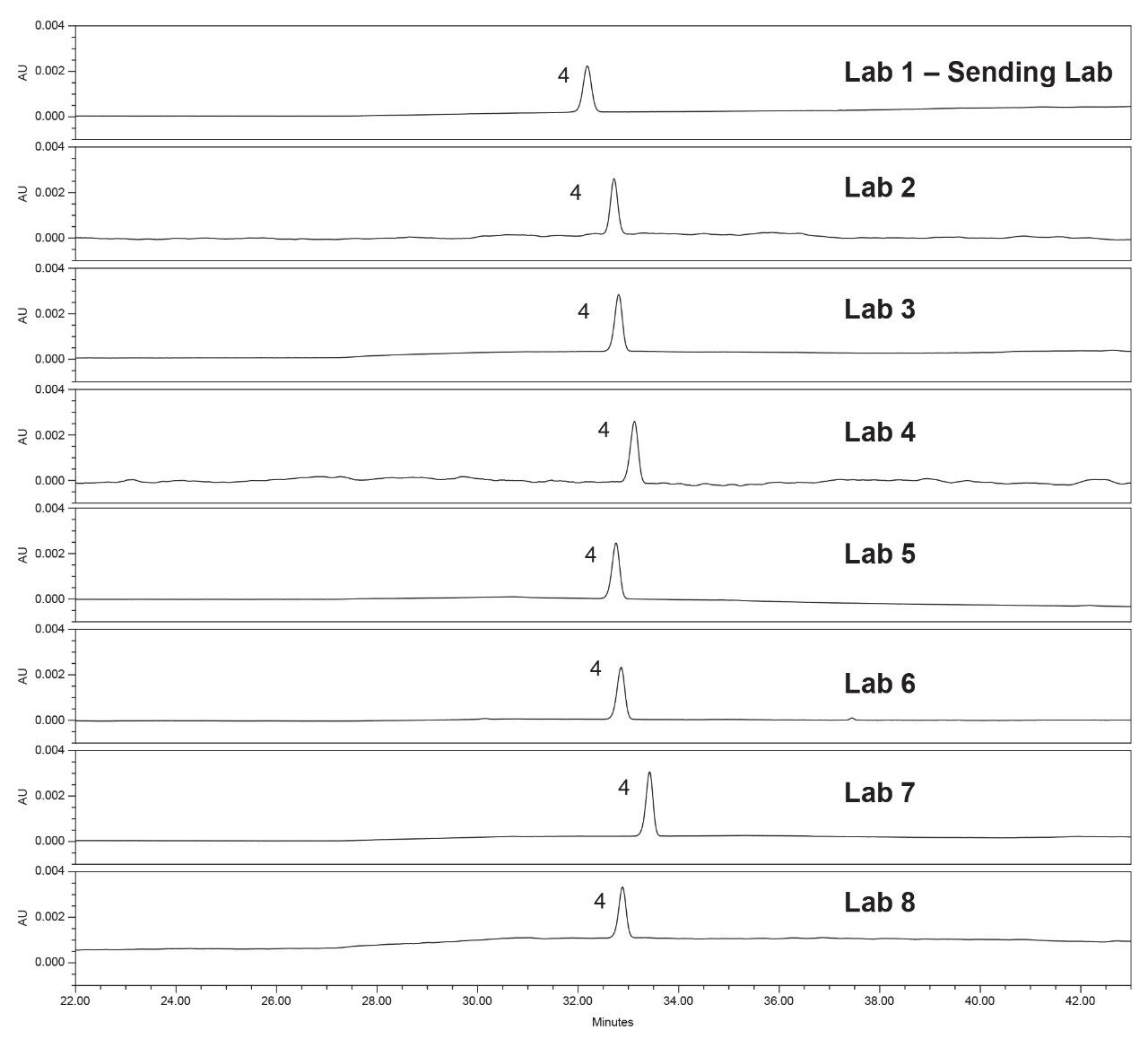
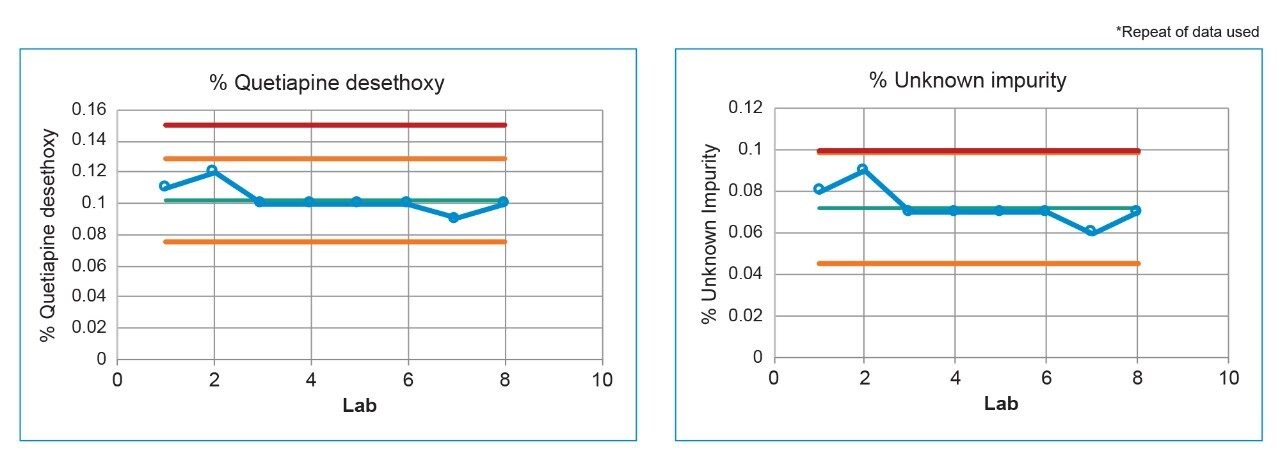
Once all the instrumentation met the system suitability requirements, the sample solution or drug substance was analyzed for the presence of impurities. The quantitative analysis showed the presence of quetiapine desethoxy (verified by the standard) and an unknown impurity. The retention times were within the expected variation of +/-3 standard deviations from the mean (Figure 6) and all the labs had comparable quantitative results of the quetiapine desethoxy and the unknown impurity found in the sample solution. All results were within the acceptance criteria of not more than 0.15% for the quetiapine desethoxy, and not more than 0.10% for the unknown impurity. Since the drug substance was past the expiration date, the presence of an impurity was not surprising. Comparison of the control charts shows that the quantitative results were within +/-3 standard deviations, indicating a state of control. Furthermore, the control limits were less than or equal to the limit specified in the USP method, giving greater confidence that the drug substance met the criteria.
The USP impurities method for quetiapine fumarate was successfully transferred to Arc HPLC instruments in eight global laboratories using a risk-based approach. Understanding the configuration of the liquid chromatography system enabled control strategies to be put into place and provided greater control and increased the likelihood of meeting system suitability criteria. Furthermore, understanding the risk of the method performance and implementing control strategies led to improved reproducibility. Through this interlaboratory study we were able to demonstrate the ability to successfully replicate USP monographs globally on the Arc HPLC System
Contributors: Margaret Maziarz, Bheeshmacharyulu S, Tian Chi Yang, Sharon Fang, Rosana Jimenez, Melanie Richards and Baris Orgun*
Waters Corporation, Milford, MA and other locations *LiKrom Analytical Solution Marketing Co., Inc, Istanbul, Turkey
720007285, June 2021