Analytical Considerations for Extractables Screening With Liquid Chromatography-High Resolution Mass Spectrometry
Abstract
The screening of components for potential extractable and leachable (E&L) compounds in pharmaceutical packaging, food contact materials, medical devices, and other consumables across various industries, is becoming more and more important due to increasing global regulations. To ensure consumer safety and reduce the risk from these components it is important to identify any potentially harmful compounds.
Liquid chromatography coupled to high resolution mass spectrometry (LC-HRMS) is the technique typically employed to analyze non-volatile organic compounds. The screening method utilized needs to cover a wide diversity of chemicals and be sensitive to low levels of these compounds. Whilst there is some guidance in the regulations with respect to sample extraction techniques, there are currently no prescribed guidelines when it comes to developing the screening methodology on an analytical instrument. It is down to the analyst to design and implement their own LC-HRMS screening method to analyze their components for potential E&L compounds.
The aim of this study was to evaluate existing E&L screening methodologies and their impact on typical E&L type compounds and assess a suitable combination of method conditions for a first-pass generic screening method. Also described are some additional considerations that can be made when designing an LC-HRMS screening method, for the analysis of extractables and leachables.
Benefits
- Tips and considerations for designing an initial generic LC-HRMS method for extractables and leachables screening applications
- Sensitive first-pass extractables screening method to cover a wider chemical diversity
- Simple LC-MS methodology with high resolution mass spectrometry for cosmetics, food contact materials, pharmaceutical packaging, and medical devices extractable applications
Introduction
The screening of components for potential extractable and leachable (E&L) compounds in pharmaceutical packaging, food contact materials, medical devices, and other consumables across various industries, is becoming increasingly more important due to growing global regulations.1,2 To ensure consumer safety and reduce the risk from these components, it is important to identify any potentially harmful compounds present.
When considering the manufacturing process of these components, there are several steps along the way from starting materials to final product and each of these steps can introduce additional impurities and degradation products, providing potential for E&Ls. These components can be very complex and can contain a wide diversity of chemicals and thus a range of analytical techniques are needed for screening and characterization. Liquid chromatography coupled to high resolution mass spectrometry (LC-HRMS) is the technique typically employed to analyze non-volatile organic compounds. The screening method utilized needs to cover the wide chemical diversity and be sensitive to low levels of these compounds.
While there is some guidance in the regulations with respect to the sample extraction techniques, there are currently no prescribed guidelines when it comes to developing the screening methodology on these analytical instruments.3,4,5 It is down to the analyst to design and implement their own LC-HRMS screening method to analyze their components for potential E&L compounds.
The aim of this study was to evaluate existing in-house E&L screening methodologies and their impact on typical E&L type compounds and assess a suitable combination of method conditions for a first-pass generic screening method. The method described herein is not an optimized method but has been developed using a limited number of solvents, additives, and method conditions based on a range of existing E&L screening methodologies. This was to find the best in-house solution for a generic E&L screening method, with a potentially large number of analytes and chemistry types, where the target list of analytes is not necessarily known. For a specific set of analytes, with a specific chemistry, a more targeted and optimized method is advised. Also described are some additional considerations that can be made when designing an LC-MS screening method for the analysis of E&L.
Experimental
Analysis Protocol
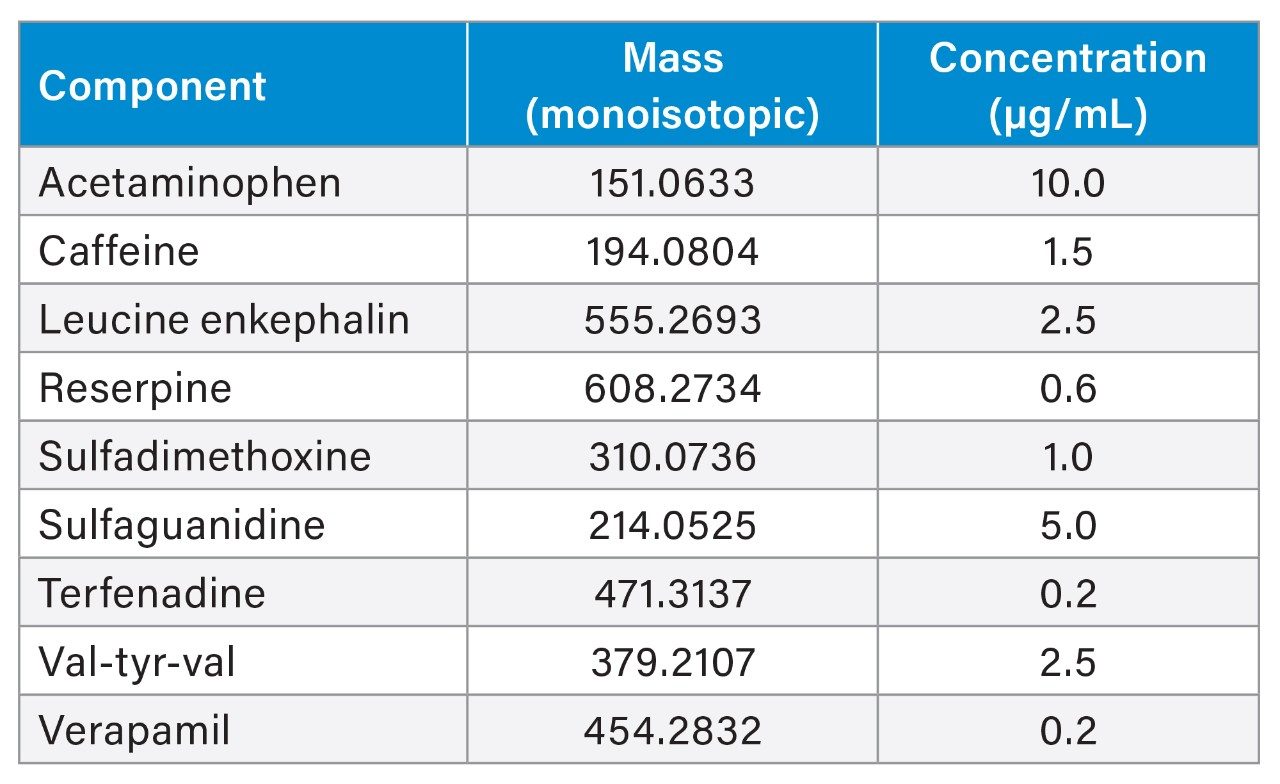
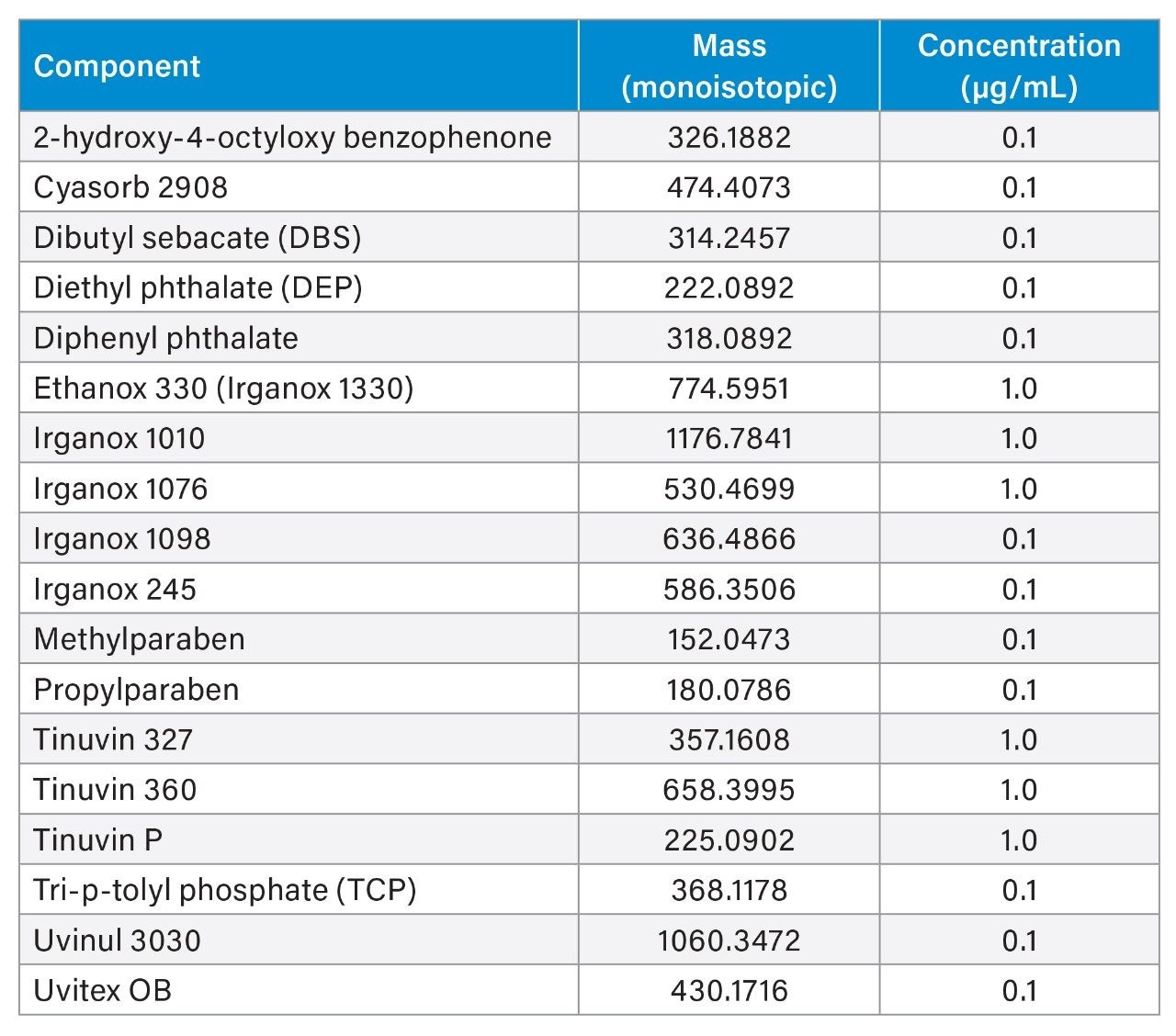
The system underwent a thorough cleaning protocol with acid, base, and IPA washes to minimize contamination and background noise before injections of standards. To assess different compound types, two standard mixes, the Waters LC-MS QC reference standard (LC-MS mix) and the Waters E&L screening standard (E&L mix), were injected to cover a range of polarities, masses, and compound chemistries that ionize in both negative and positive ESI (electrospray ionization) modes (Tables 1 and 2). Blank injections of methanol were run before triplicate injections of each standard, in both positive and negative ionization modes.
LC Conditions
|
LC system: |
ACQUITY UPLC I-Class FTN |
|
Column(s): |
CORTECS UPLC C18 Column, 90 Å, 1.6 µm, 2.1 x 100 mm (Waters p/n: 186007095) |
|
Column temp.: |
40 °C |
|
Injection volume: |
1 µL |
MS Conditions
|
MS system: |
IMS QTof Mass Spectrometer |
|
Collision gas: |
Argon |
|
Ionization mode: |
ESI+, ESI- |
|
Acquisition mode: |
HDMSE |
|
Acquisition range: |
50–1500 m/z |
|
Scan time: |
0.2 seconds |
|
Source temperature: |
120 °C |
|
Desolvation temperature: |
500 °C |
|
Desolvation gas: |
800 L/hr |
|
Cone gas: |
50 L/hr |
|
Reference mass: |
Leucine enkephalin [M+H]+ m/z 556.2766 Leucine enkephalin [M-H]- m/z 554.2620 |
|
Capillary voltage: |
ESI+, ESI- 1 kV |
|
Collision energy: |
ESI+ low energy: 6 eV high energy ramp: 20–40 eV ESI- low energy: 6 eV high energy ramp: 30–70 eV |
Data Management
|
Chromatography software: |
UNIFI Scientific Information System v1.9.4 |
|
MS software: |
UNIFI Scientific Information System v1.9.4 |
|
Informatics: |
UNIFI Scientific Information System v1.9.4 |
Methodologies
Three mobile phase combinations and three gradients were evaluated and compared:
Mobile Phases
|
Mobile Phase Combination 1 (MPC 1) |
|
|
Mobile phase A: |
Water + 0.1% formic acid |
|
Mobile phase B: |
Methanol + 0.1% formic acid |
|
Mobile Phase Combination 2 (MPC 2) |
|
|
Mobile phase A: |
Water + 1 mM ammonium acetate + 0.1% acetic acid |
|
Mobile phase B: |
Methanol |
|
Mobile Phase Combination 3 (MPC 3) |
|
|
Mobile phase A: |
Water + 1 mM ammonium acetate + 0.1% formic acid |
|
Mobile phase B: |
Methanol + 1 mM ammonium acetate + 0.1% formic acid |
Results and Discussion
Gradient Comparison
The three different gradient conditions were evaluated with each of the mobile phase combinations. Similar trends were seen across the different mobile phases, therefore the results highlighted here are from Mobile Phase Combination 2 (MPC 2) and in positive ionization mode.
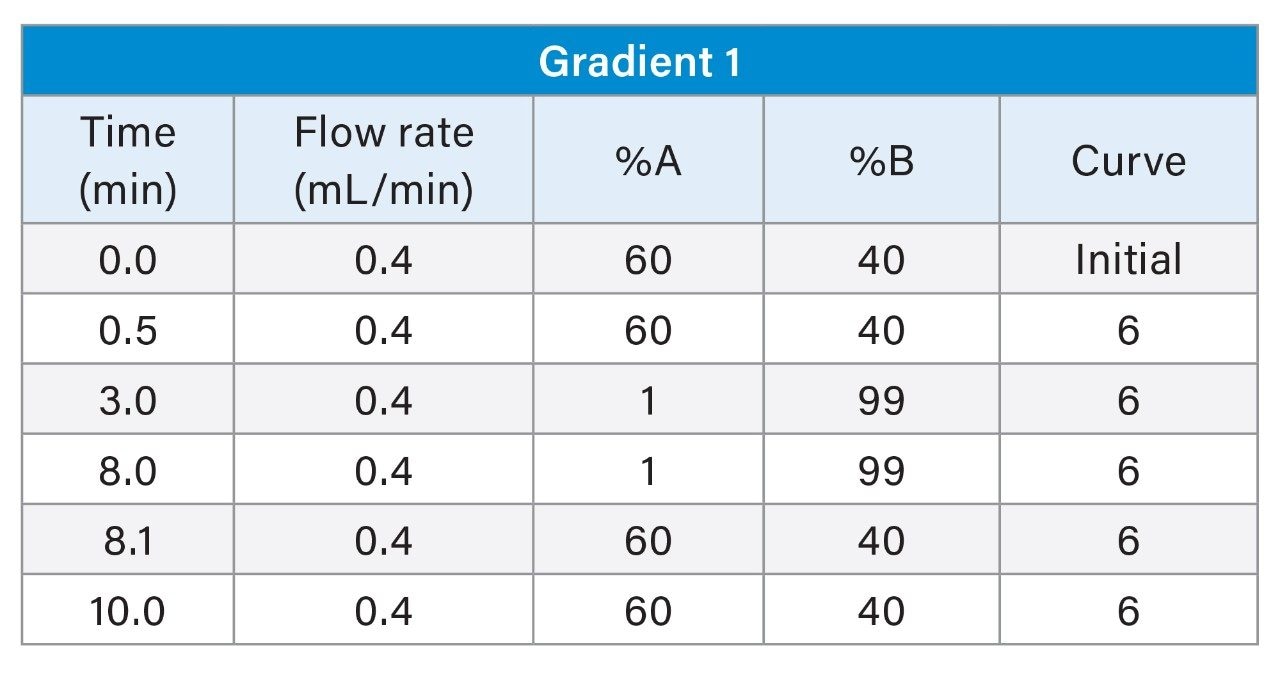
1. Gradient 1
The LC chromatograms for Gradient 1 with MPC 2 for the LC-MS mix (Figure 1) and the E&L mix (Figure 2) are highlighted here. For the LC-MS mix, polar analytes elute very early from the column, which poses the risk of not detecting some analytes that elute in the void volume. These early eluting analytes are also poorly separated. For the E&L mix, even if good peak shapes are observed, many of the analytes elute in a four-minute window which will be counter productive in complex mixtures.
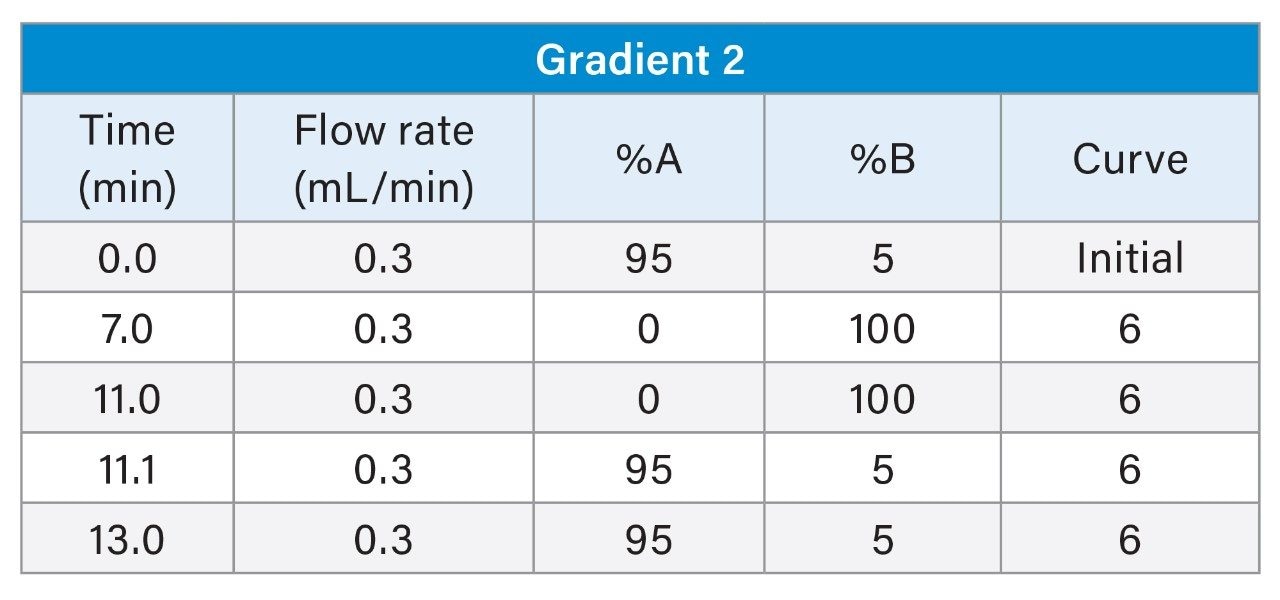
2. Gradient 2
The LC chromatograms for Gradient 2 with MPC 2 for the LC-MS mix (Figure 3) and the E&L mix (Figure 4) are highlighted here. For the LC-MS mix, the more polar analytes are now eluting from the one-minute mark with better separation for these analytes. As this gradient starts at a very low organic mobile phase percentage, it increases the number of different types of chemistries covered, which is more suitable for an initial generic screening method. For the E&L mix, many of the analytes elute in a three-minute window, which compromises separation and may affect detection of some analytes in complex mixtures due to competition for charges.
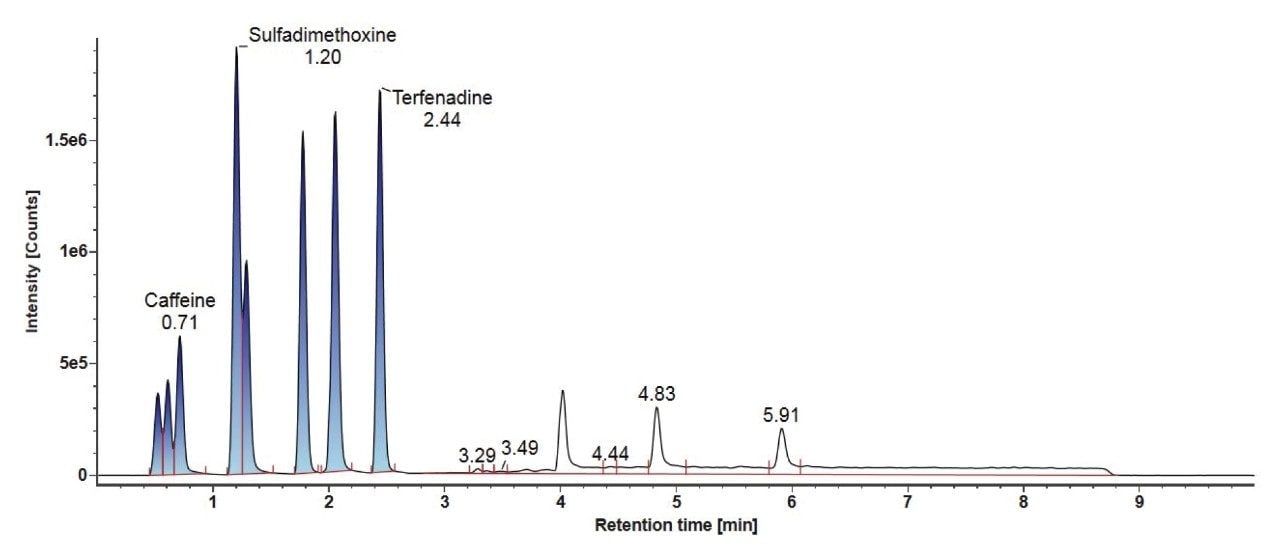
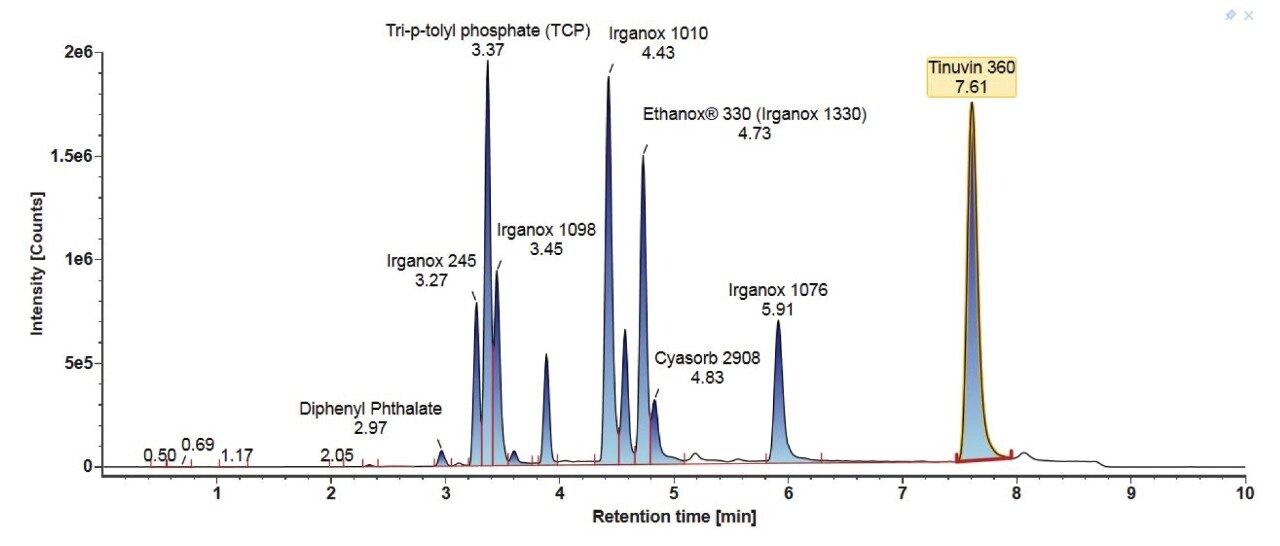
Consideration needs to be taken for non-polar analytes, as observed below in the case of Tinuvin 360 (Figure 4). In the E&L mix, Tinuvin 360 should be the last eluting compound due to its polarity, however, Irganox 1076 is observed as the last eluting analyte with Tinuvin 360 not being observed at all. A possible cause for this observation is that the organic hold in this gradient is too short and there is no time for the analyte (Tinuvin 360) to elute from the column. Subsequent injections of the E&L mix showed this is the case with Tinuvin 360 eluting earlier than expected in the second injection (Figure 5). This is an important point to consider during method development, as not only can an analyte be missed, but the retention time can be incorrectly recorded for targeted screening affecting the identification of E&L compounds.
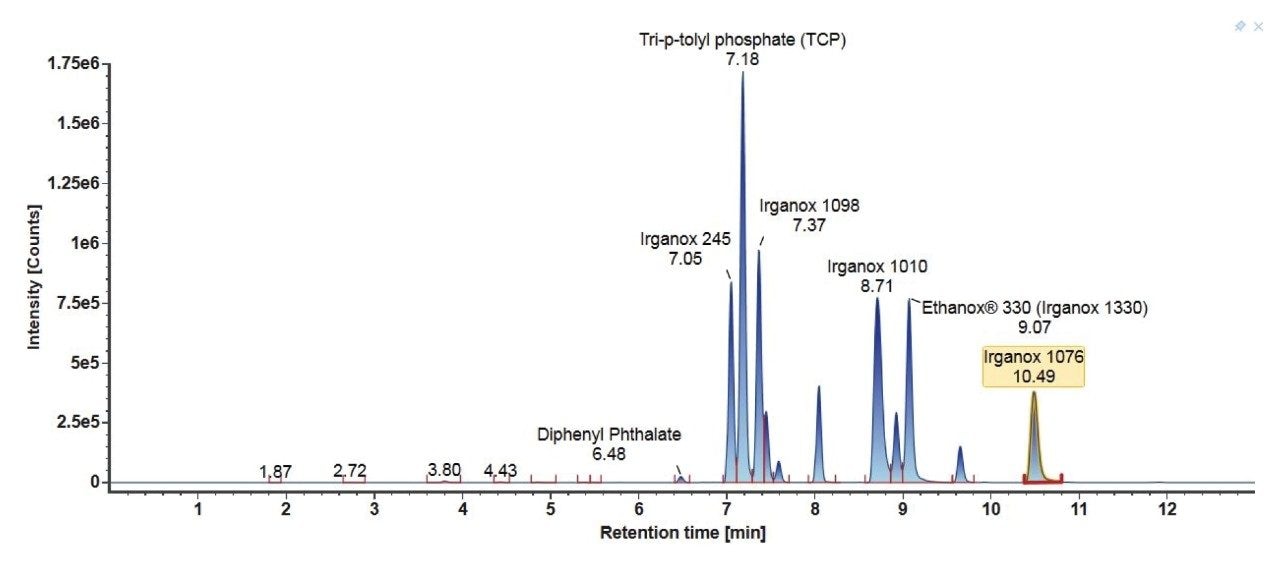
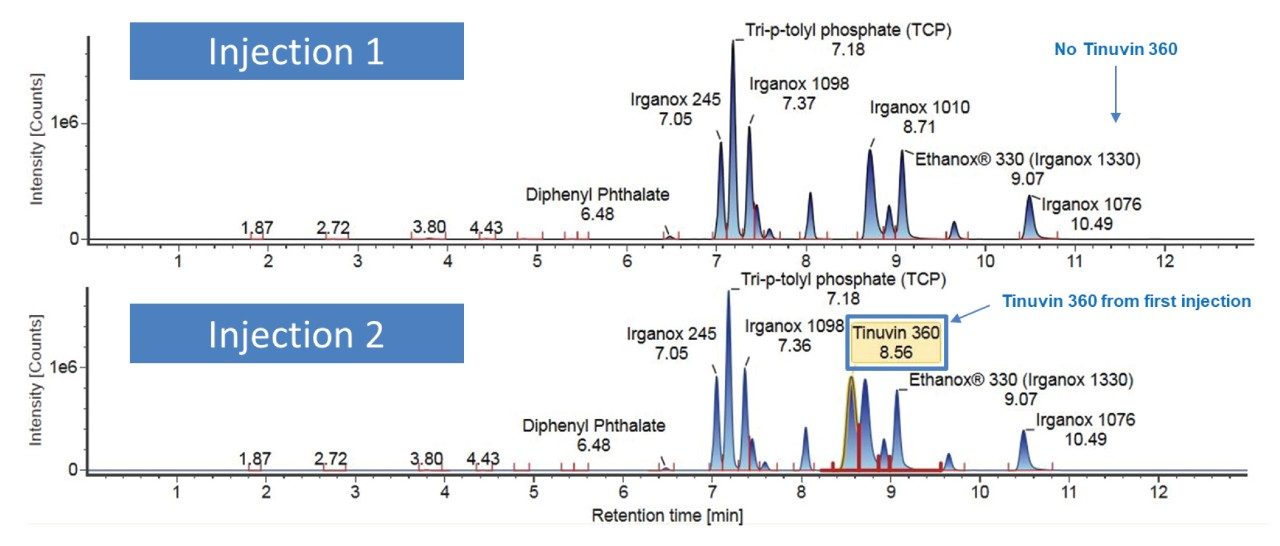
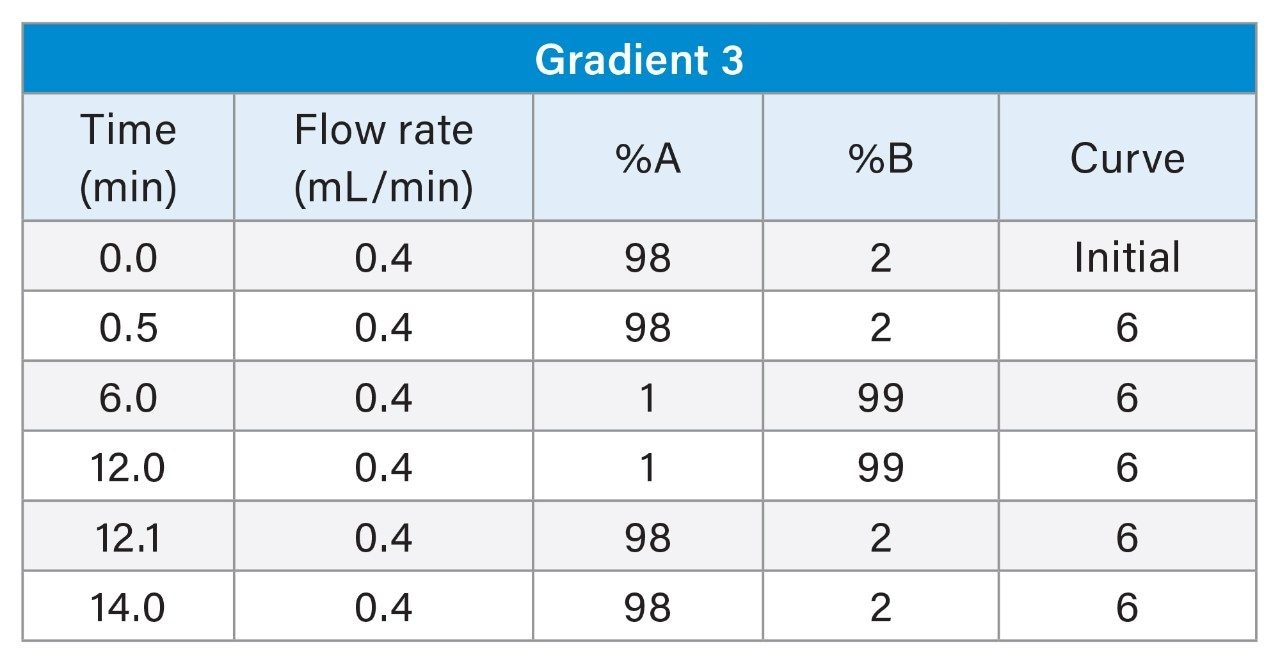
3. Gradient 3
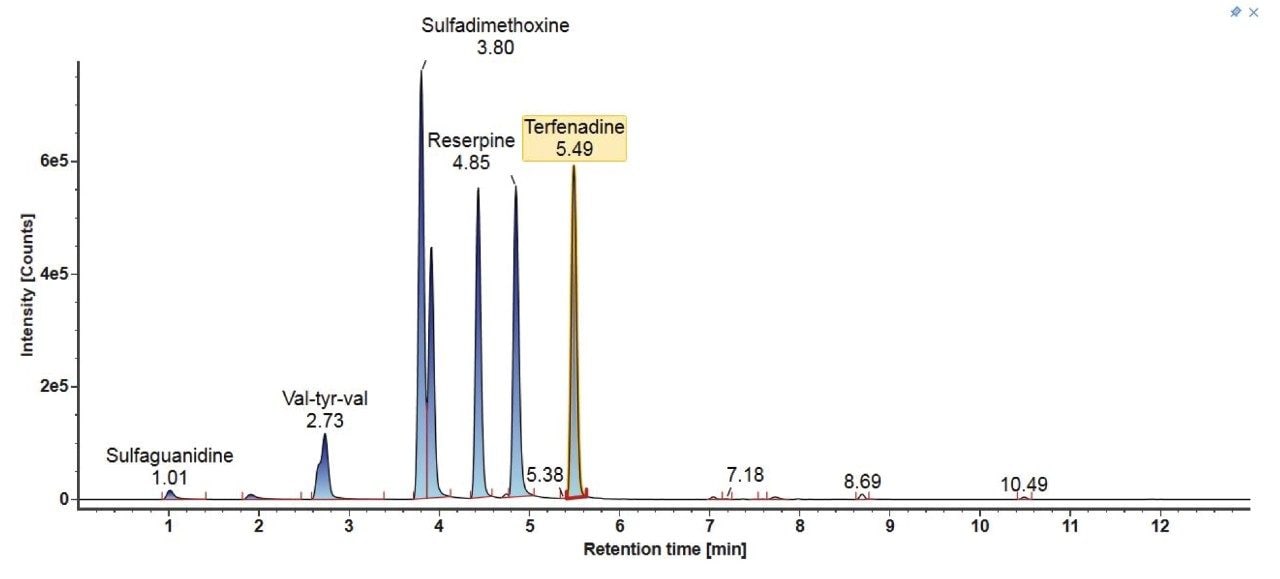
The LC chromatograms for Gradient 3 with MPC 2 for the LC-MS mix (Figure 6) and the E&L mix (Figure 7) are highlighted here. For the LC-MS mix, similar to Gradient 2, the starting organic mobile phase percentage is lower, therefore the more polar analytes are better retained on the column and better separation is observed. For the E&L mix, most analytes are now eluting in a larger window with better separation. The organic hold is long enough to ensure that Tinuvin 360 is the last eluting compound in all injections.
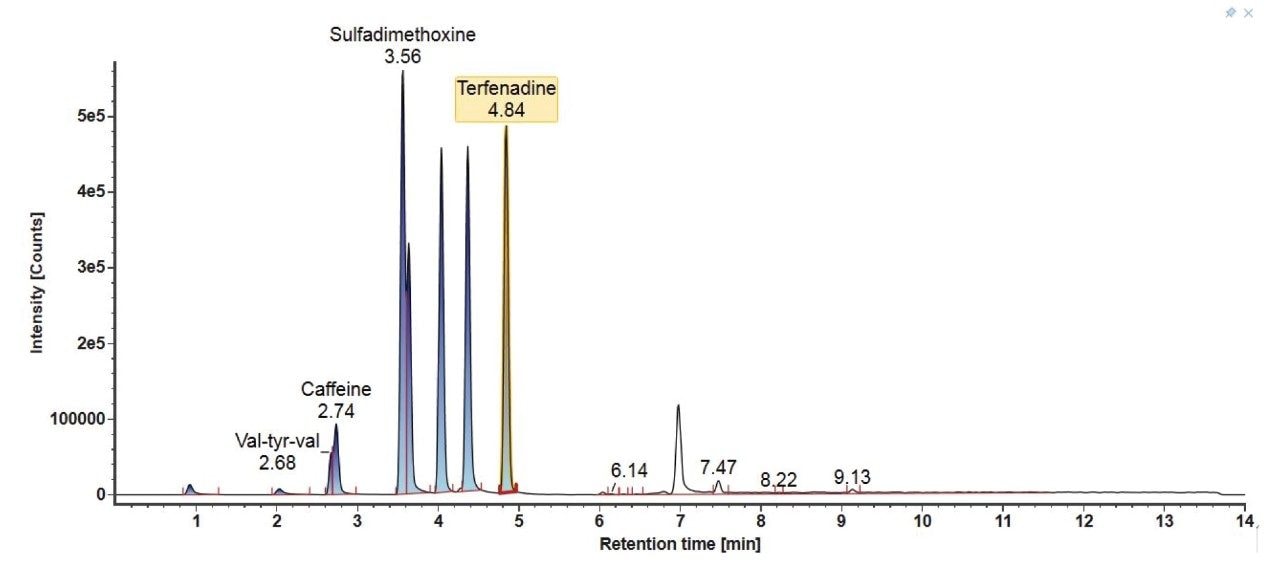
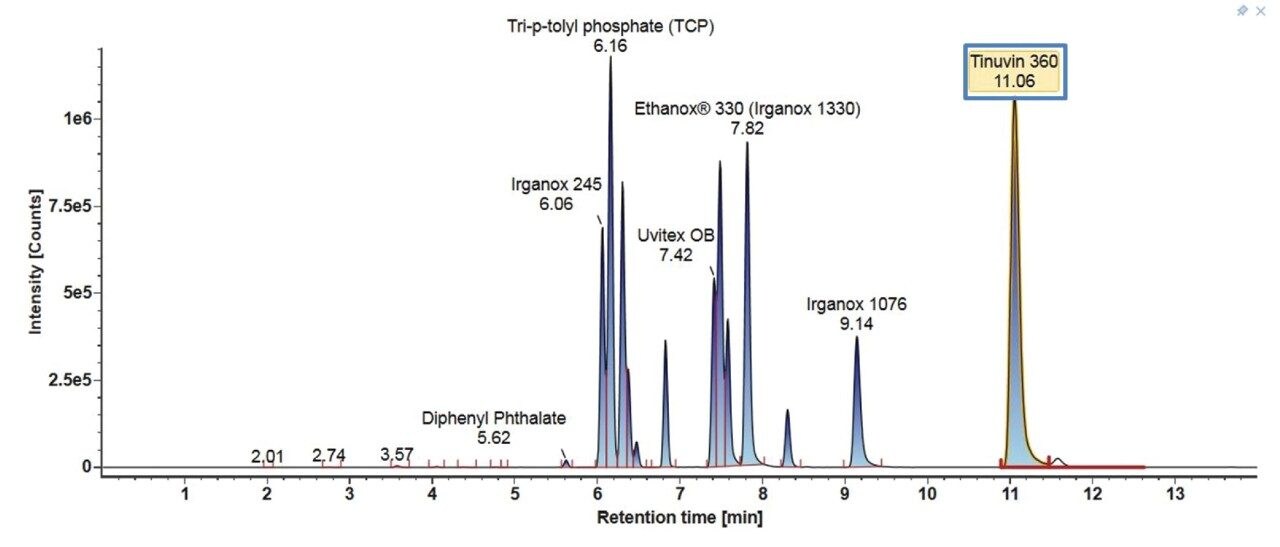
4. Gradient Comparison Summary
From these experiments, we can conclude that to develop a generic E&L screening method, a lower percentage of organic mobile phase in the gradient starting conditions and an organic mobile phase hold at the end of the gradient are critical for the detection of a wide range of analytes. These factors are coupled together in Gradient 3, giving a good level of separation across the whole range of compounds, whilst the risk of missing analytes; either in the void volume or through over retention on the column, is reduced.
Mobile Phase Comparison
The three different mobile phase combinations were evaluated with each of the three different gradients. Most analytes showed similar results across the different gradients, therefore the results highlighted here are from Gradient 3. The results are represented in charts that show the average MS (mass spectrometric) response for each analyte across the triplicate injections for each mobile phase combination. Mobile Phase Combination 1 (MPC 1) is highlighted in pink, Mobile Phase Combination 2 (MPC 2) is highlighted in blue, and Mobile Phase Combination 3 (MPC 3) is highlighted in green.
1. Positive mode
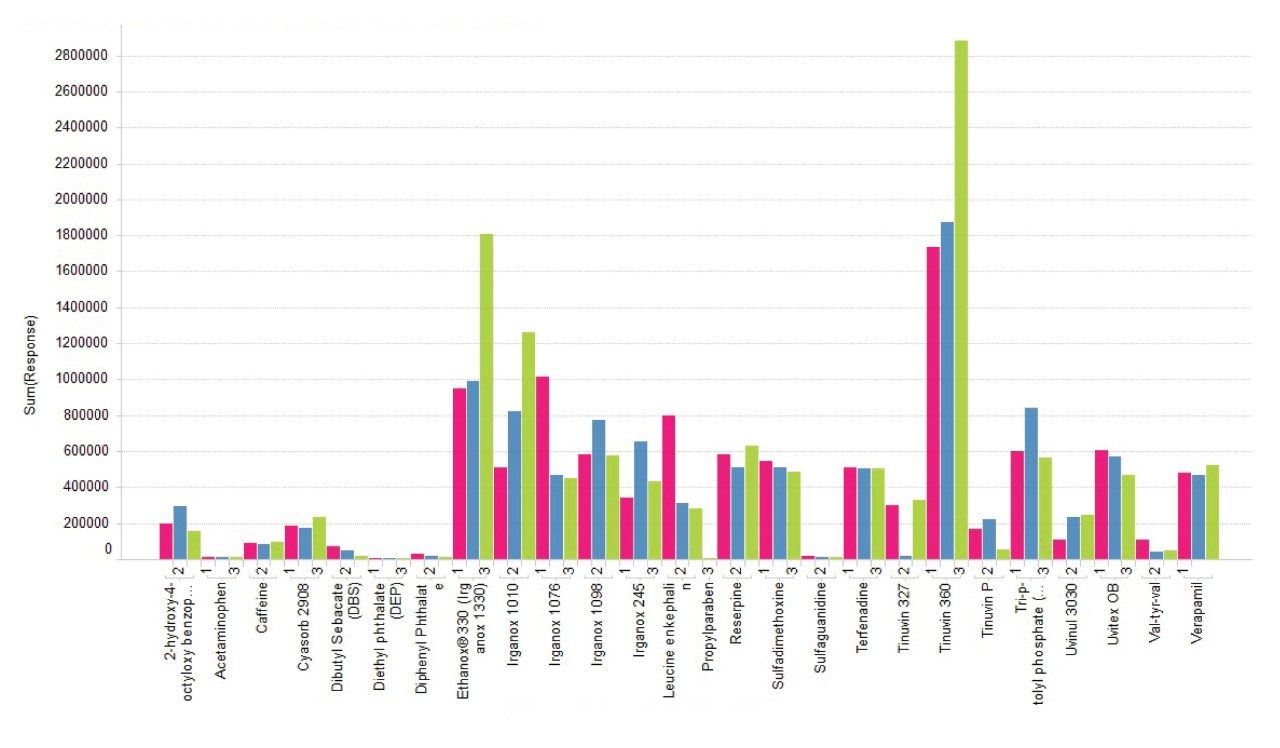
A graph showing positive ionization responses for all the analytes in the LC-MS and E&L mixes using the three mobile phase combinations as described in the experimental section are shown in Figure 8. Most of the analytes show a slight change across the mobile phase combinations with a selection of the E&L mix analytes showing an increased response with MPC 2 or MPC 3 in positive mode.
2. Negative mode
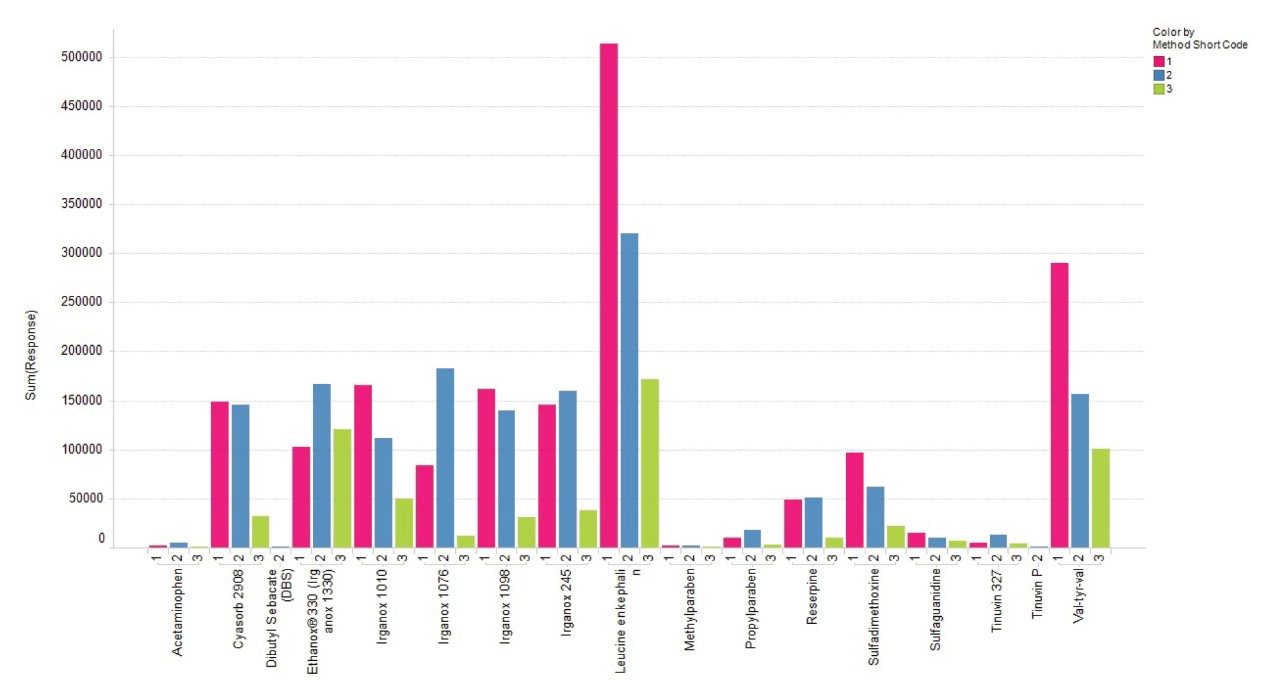
Mobile phase combinations for the LC-MS mix and the E&L mix analytes in negative ionization mode are shown in Figure 9. MPC 1 and MPC 2 show an increased response across the range of analytes in negative mode compared to MPC 3.
3. Mobile Phase Comparison Summary
The mobile phase comparison shows the effect of mobile phase composition on the ionization of the analytes. For an initial generic screening method, it is worth finding additives that do not have a strong bias for certain analyte types. From the data shown above, Mobile Phase Combination 2 gave the best average response across most analytes in both positive and negative ionization modes.
Figures 8 and 9 show the combined response for all adducts observed for each analyte, thus it is important to consider adduct formation in the ionization of extractable compounds as a selection of analytes may have a stronger response when an additive is used in the mobile phase. Depending on the chemical structure of the extractable compounds, the formation of ammonium adducts may be favorable when mobile phases containing additives like ammonium acetate are used.
The addition of additives like ammonium acetate to the mobile phases can lead to the formation of different types of adducts, [M+NH4]+, [M+Na]+, which will have to be considered in the total response of the analytes as these can be more intense than the protonated species.
Additional Considerations
After the investigation and comparison work, Gradient 3 and Mobile Phase Combination 2 were observed to give the best compromise between good chromatographic separation and MS responses across the range of analytes and the different ionization modes. Some additional considerations to further improve the method for a generic E&L screening application are discussed here.
1. Solvent Evaluation
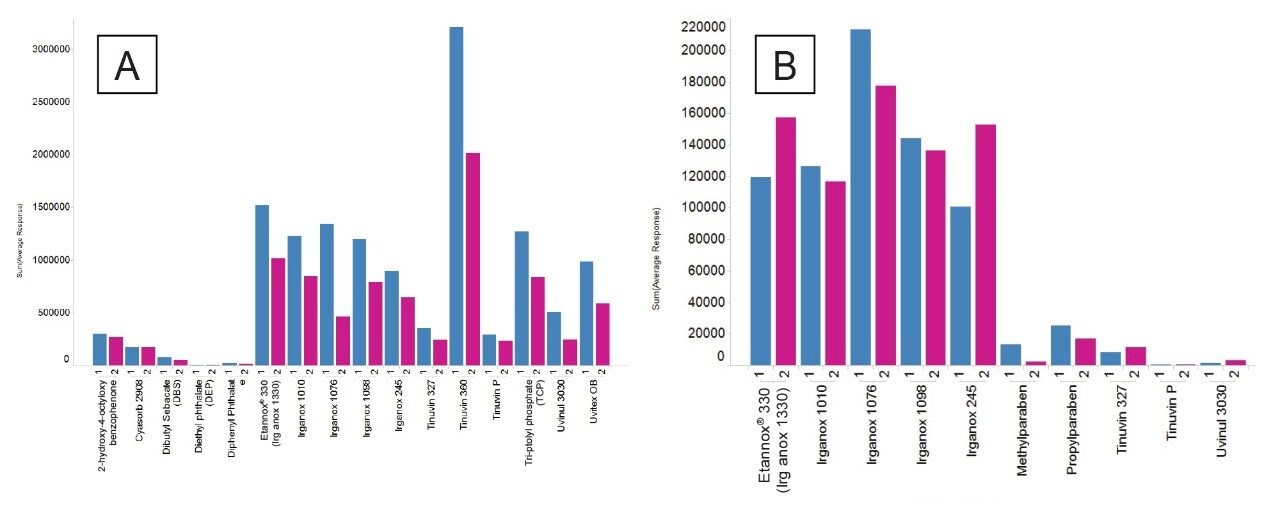
It is important to consider the quality of solvents and additives to help reduce the background matrix in the LC chromatograms when preforming E&L screening studies. Furthermore, it is also important to assess the strength of additives. Formic acid is a stronger acid than acetic acid and better for ionization (acetic acid pKa ~4.8 and formic acid pKa ~3.8, same concentration of the acid in the mobile phase will give you lower pH in the case of formic acid) and after further evaluation using the E&L mix it was found to show a slight increase in responses for the majority of the E&L analyte mixture when used as a replacement additive in mobile phase A in MPC 2 (Figure 10).
2. Pressure Variations
Often, E&L screening methods are transferred across different LC systems which might vary in pressure limits. As the organic mobile phase used is methanol, which is of higher viscosity than acetonitrile, to avoid over-pressuring systems with lower pressure limits the flow rate was dropped, and the column temperature increased to mitigate this effect. With the lower flow rate, the organic hold was further increased to account for this change.
3. Final Method Conditions
After evaluation of the existing LC-MS screening methodologies and some further considerations, the final generic screening conditions selected for in-house E&L LC-HRMS screening are highlighted below.
UPLC Conditions
|
Column: |
Waters CORTECS UPLC C18 Column, 90 Å, 1.6 µm, 2.1 x 100 mm (p/n: 186007095) |
|
Column temperature: |
50 °C |
|
Injection volume: |
1 µL |
Mobile Phase Combination
|
Mobile Phase Combination |
|
|
Mobile phase A: |
Water + 1 mM ammonium acetate + 0.1% formic acid |
|
Mobile phase B: |
Methanol |
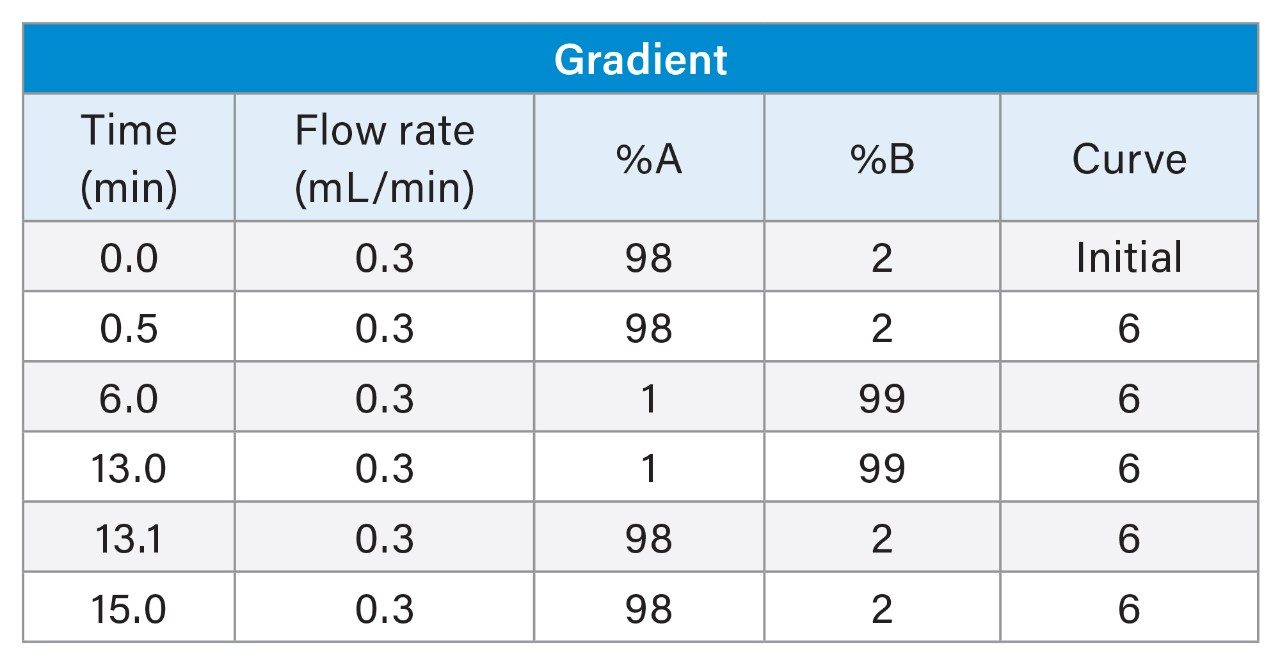
Gradient
Conclusion
An initial first-pass, generic E&L screening method has been evaluated. It is important to consider that the method described was developed from a combination of already existing methodologies and it was never the aim of this study to produce an analysis method from first principles. Thorough investigation and comparison work were undertaken to evaluate the impact of the varying conditions on different chemistry types. Further considerations were made to create a single, generic screening method to support E&L analysts.
The method developed from the experiments described minimized risk of carryover of late eluting compounds whilst increasing the chromatographic resolution of early retained compounds. Further changes allowed the method to be transferred across different LC systems with varying pressure limitations, while also considering solvent strength. It showed the best overall sensitivity across a large polarity range and was without bias towards certain chemistry types.
In general, the following key observations were demonstrated that can be considered when developing and streamlining an LC-MS screening method for E&L:
- When creating the chromatographic gradient for a generic screening method, the starting organic percentage and the length of the organic hold are important to capture a wide range of analyte polarities, whilst minimizing the risk of losing analytes, due to not retaining or retaining too much to the column.
- When choosing mobile phases, opting for a combination of solvents, additives, and buffers that do not show bias towards a particular set of analytes is a good starting point for an initial generic screening method. Using buffers in your mobile phase might help to enhance the response of some typical extractables whilst also reducing the formation of less favourable adducts.
Taken together, this application note describes suggestions for developing a generic initial screening method for E&L. However, if the method is to be employed for a specific set of analytes, or where knowledge of the types of chemistries present is known, then further optimization is recommended. Different mobile phases for positive and negative ionization modes, and different additives, can be utilized for a more targeted screening method.
References
- Food and Drug Administration, 2000, Code of Federal Regulation Chapter 21.
- Official Journal of the European Union, 2011, Regulation 10/2011/EU.
- Food and Drug Administration, 2015, Analytical Procedures and Methods Validation for Drugs and Biologics Guidance for Industry https://www.fda.gov/files/drugs/published/Analytical-Procedures-and-Methods-Validation-for-Drugs-and-Biologics.pdf.
- Norwood, D.L., Paskiet, D., Ruberto, M. et al. Best Practices for Extractables and Leachables in Orally Inhaled and Nasal Drug Products: An Overview of the PQRI Recommendations. Pharm Res 25, 727–739 (2008). https://doi.org/10.1007/s11095-007-9521-z.
- ISO 10993–18:2020 Biological evaluation of medical devices–Part 18: Chemical Characterization of Medical Device Materials within a Risk Management Process, https://www.iso.org/standard/64750.html.
Featured Products
720007492, January 2022