Extractables, Leachables, and Contact Materials: The Invaluable Benefit of Ion Mobility-Enhanced Mass Spectrometry Libraries
Abstract
In collaboration with Merck KGaA, a set of extractables and leachables certified reference materials (CRM) mixes were used to produce a ultraperformance liquid chromatography ion mobility-mass spectrometry (UPLC-IM-MS) library, comprising retention time, precursor ion, product ion m/z, and collision cross section (CCS) values in ES+ and ES- modes. Ion mobility-enhanced mass spectrometry libraries incorporate additional cumulative specificity compared to conventional mass spectrometry libraries. They can be used to reduce false detection rates and increase confidence of identification in complex matrices.
A non-targeted screening approach was performed utilising the UPLC™-IM-MS library to screen two food commodity samples. Ubiquitous components of plastics, natural constituents and spiked analytes were identified using the extractable and leachable (E&Ls) library. When experimental outcomes were compared to the mass spectrometry library data, ΔCCS values were within 2% and retention times withing 0.1 minutes, allowing identifications to be confidently made. CCS values provided added identification confidence where product ions were not observed.
Verification of the E&Ls library generated has been performed and the library can facilitate non-targeted screening to identify E&Ls.
Benefits
- An extractables and leachables ion mobility enhanced mass spectrometry library inclusive of CCS values has been generated. The library affords additional specificity compared to conventional mass spectrometry libraries
- Reduced false detections and increased confidence of identification in complex matrices
- The retention time, precursor ion and product ion information can be applied in assays incorporating conventional MS data
Introduction
Identification and characterization of E&L components in various industries are being driven by increasing global regulations.1–7 Product packaging or contact materials are made of a wide variety of chemicals, which can include polymers and polymer additives such as antioxidants, UV-stabilisers, slip agents, and colorants. These chemicals, their impurities, and degradation products can migrate into consumer products, adding unwanted and potentially harmful substances into food, medicine, and cosmetics. The breadth of man-made products to consider is vast, ranging from a simple food label to recycled packaging, clothes, drug delivery systems, and implantable medical devices. A typical E&L analysis workflow initially starts with a targeted screening step. The screening is based on a library or database of components where matches are made against accurate mass, retention times, and product ion data. The library quality is critical for a reliable screening application. The next workflow step is non-targeted screening, with subsequent characterization of any unknown components found. This step is typically complex and time-consuming; however, comparison and elucidation software tools can aid and accelerate the process.
High resolution mass spectrometers (HRMS) such as quadrupole time-of-flight mass analysers (Q-ToF), have become more prevalent as screening tools. Using non-targeted “full spectra” data acquisition thousands of detections can be made in a single analysis and can be followed by retrospective targeted data analysis. The drive for higher sample throughput is global, with requirements for improved time efficiency and cost reduction resulting in movement towards multiclass compound analysis. This approach has been used to analyse food and environmental samples, as well as organic contaminants which reside within a variety of complex sample matrices.8–13 The purpose of a screening method is to rapidly detect and identify target compounds in the sample under investigation, with false detection rates being kept as low as possible. Using measured properties of a compound, such as the accurate mass, isotope pattern, and product ion spectrum, appropriate filters can be applied to determine the presence of a compound in a sample. Within complex matrices, using these properties alone to achieve matrix or analyte identification may prove to be more challenging and additional method development strategies need to be employed. The extra dimension of ion mobility (IM) separation can help to mitigate such analytical challenges, as well as generate additional identification specificity via the CCS.
UPLC-IMS comprises ion mobility (gas phase separation prior to MS analysis) coupled with UPLC (neutral species separation).14,15 The timescale of UPLC (seconds), IMS (milliseconds), and time-of-flight MS (microseconds) are compatible with the requirement of high throughput analysis of complex samples. Ion mobility separation is obtained by driving packets of ions through an inert buffer gas (nitrogen) using a relatively weak electric field. The number of collisions between ions and the buffer gas results in different drift times which are a function of the ions size, shape, and charge as well as on an ion’s dipole moment in cases where polarizable buffer gas is used. Ion mobility provides an added dimension of separation to that of LC (hydrophobicity) and MS (m/z), in addition to CCS, a complementary identification descriptor. The utility of CCS to increase identification specificity has been illustrated across a wide range of applications.16–22
Using a previously described strategy for mass spectrometry library generation,23 a set of E&L compounds were characterized using UPLC-IM-MS. The library generated can be used to facilitate tandem, ToF or IM based analysis. The strategy employed provides retention time (tr), precursor ion, product ion, and CCS data for the analytes characterised.
As part of a Waters and Merck KGaA collaboration, we have jointly constructed an E&L library with CRM standard mixes used for analyte quantitation. The library of E&L compounds has been used to perform a non-targeted screen of two food commodities.
Experimental
Sample Description
Food Matrix: Orange cordial, blackcurrant with apple cordial diluted 10:1 (H2O), Sample Spiking Concentration 100pg/µL.
- Samples ES+: bis(2-ethylhexyl) phthalate, bisphenol A bis (2,3- dihydroxypropyl) ether, bisphenol A diglycidyl ether, benzyl butyl phthalate, bis(4-chlorophenyl) sulfone, dibutyl phthalate.
- Samples ES-: 3,5-di-tert-butyl-4-hydroxybenzyl alcohol, bisphenol A, 2,4-di-tert-butylphenol, 2,6-di-tert-butylphenol, terephthalic acid, octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl) propionate.
All Standards were provided by Sigma-Aldrich (Merck KGAa), Buchs, Switzerland.
Method Conditions
|
LC Conditions |
|
|
LC system: |
Waters ACQUITY™ UPLC I-Class System |
|
Vials: |
LCMS Certified Clear Glass 12 x 32mm Screw Neck Total Recovery Vial, with Cap and Pre-slit PTFE/Silicone Septa, 1 mL Volume, (p/n: 600000671CV) |
|
Column: |
ACQUITY Cortecs™ C18, 90 Å (1.6 µm, 2.1 x 100 mm Column) |
|
Column temp.: |
50 °C |
|
Sample temp.: |
6 °C |
|
Injection volume: |
10 µL |
|
Flow rate: |
0.3 mL/min |
|
Mobile phase A: |
H2O + 1 mM ammonium acetate (containing 0.1% formic acid v/v) |
|
Mobile phase B: |
MeOH |
Gradient Table
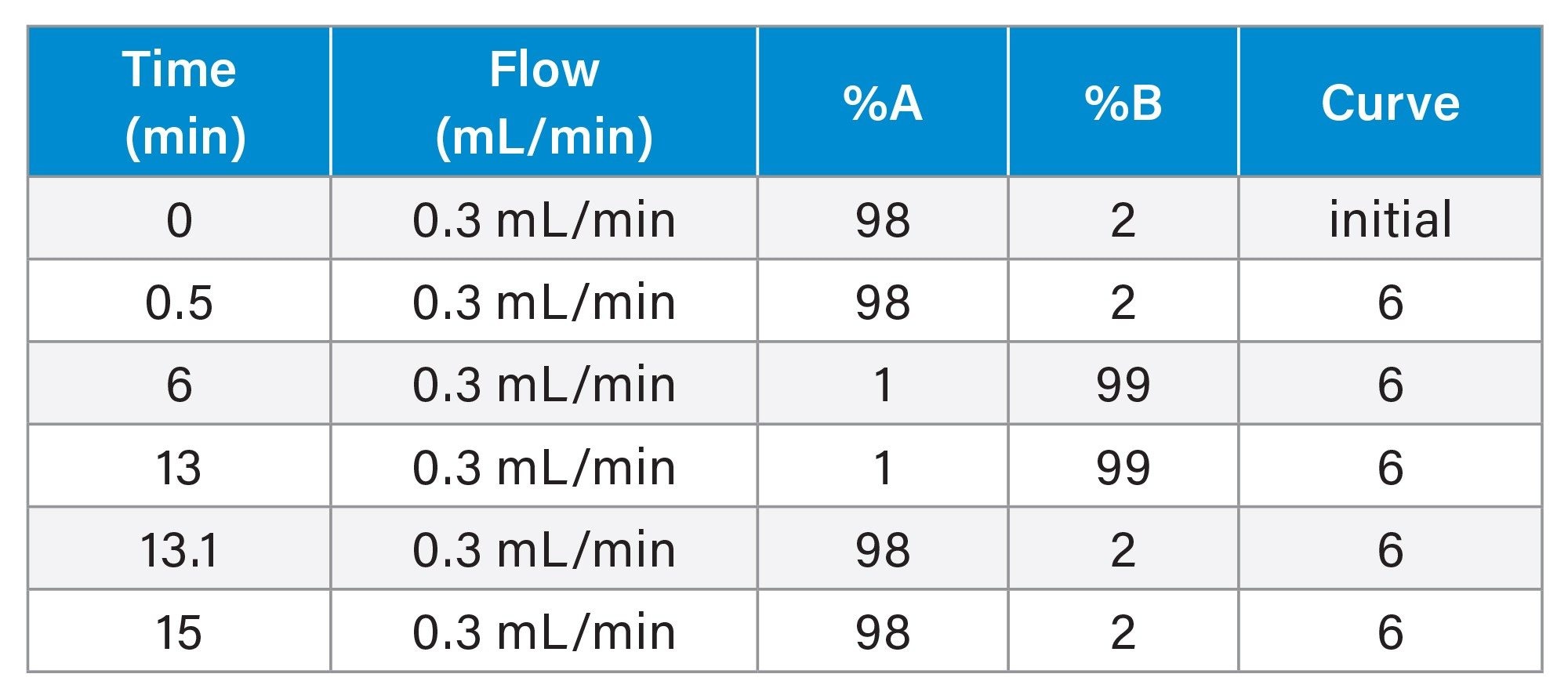
|
MS Conditions |
|
|
MS system: |
SYNAPT™ XS |
|
Ionization mode: |
ES+/ES- |
|
Acquisition range: |
m/z 50–1200 |
|
Acquisition rate: |
10 spectra per second |
|
Capillary voltage: |
1 kV/0.8 kV |
|
Desolvation temp.: |
550 °C |
|
Source temp.: |
150 °C |
|
Lock mass: |
Leucine enkephalin (m/z 556.2766) |
|
Acquisition mode: |
HDMSE |
|
Collision energy: |
Collision energy ramp (20 to 40 eV/20 to 50 eV). |
|
IMS parameters: |
Defaults include: T-Wave Velocity Ramp = Start: 1000 m/s End: 300 m/s, T-Wave Pulse Height = 40 V and a gas flow of helium 180 mL and nitrogen 90 mL (buffer gas) for the respective gas cells was used, giving an IM cell pressure of ~3.2 mBar. |
|
Calibration: |
IMS/Tof Calibration Kit (p/n: 186008113) (Waters Corp. UK). |
Results and Discussion
The field of E&L analysis is very broad and has many unknowns, however the generation of E&L libraries incorporating tr, precursor ion, product ion and CCS values provides added specificity to non-targeted screening assays. Analysis of E&Ls can be challenging due to ubiquitous contamination. The scope of the verification undertaken, was designed to verify the library and illustrate confidence in additional identifications.
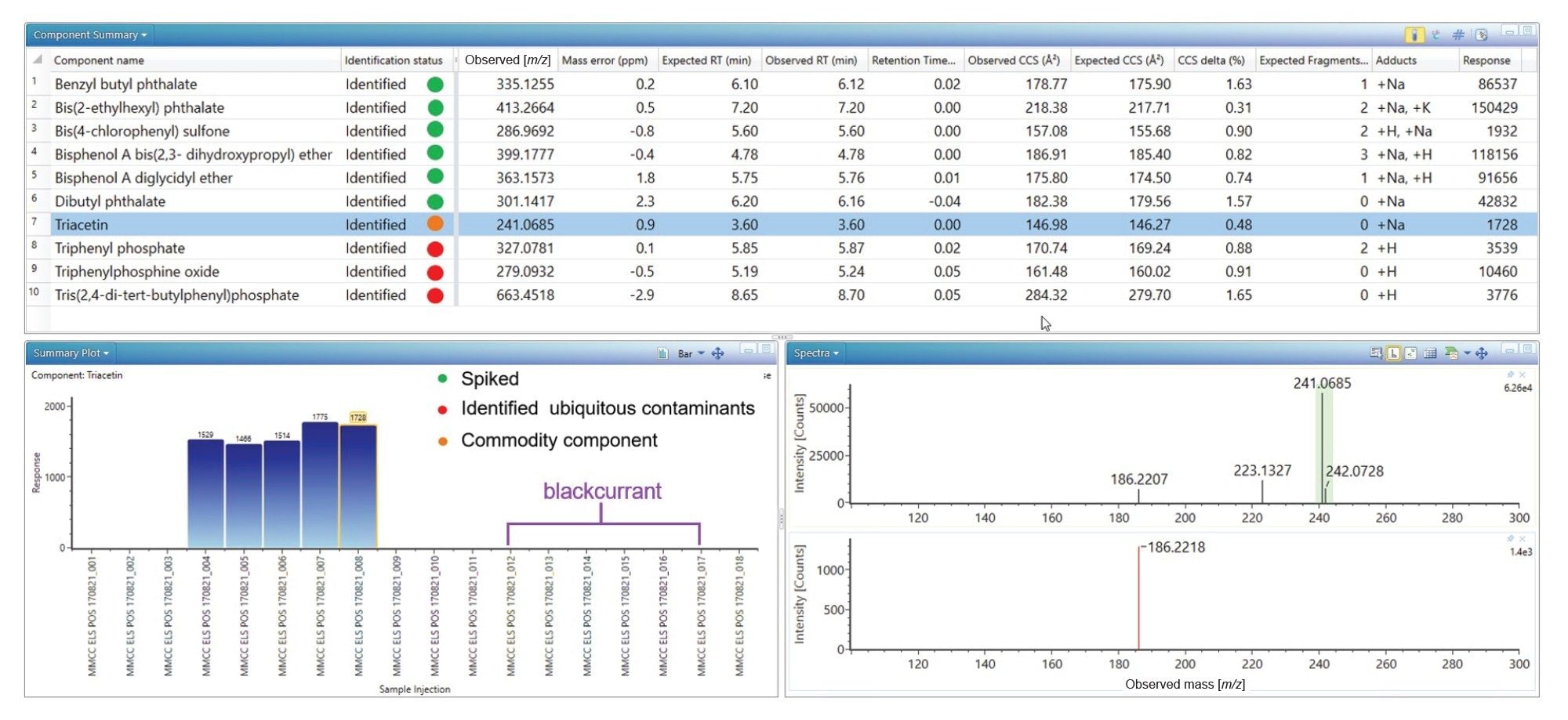
Two food commodities orange cordial and black currant with apple cordial were spiked with E&L standards at 100 pg/µL and analyzed to verify the E&L library generated. The corresponding results obtained for UPLC-IM-MS ES+ analysis of spiked orange cordial is shown in Figure 1. The spiked E&L analytes have been identified, where retention time error (<0.1 minutes), mass accuracy (<5 ppm), expected product ion count (0–3) and Δ TWCCSN2< 2% have been observed. A combination of [M+H]+, [M+Na]+, and [M+K]+ species were observed. For four of the identified analytes two TWCCSN2 values have been determined, providing increased specificity. In the case of dibutyl phthalate, no product ions were observed, however a CCS value provides an additional descriptor to enhance confidence in identification.
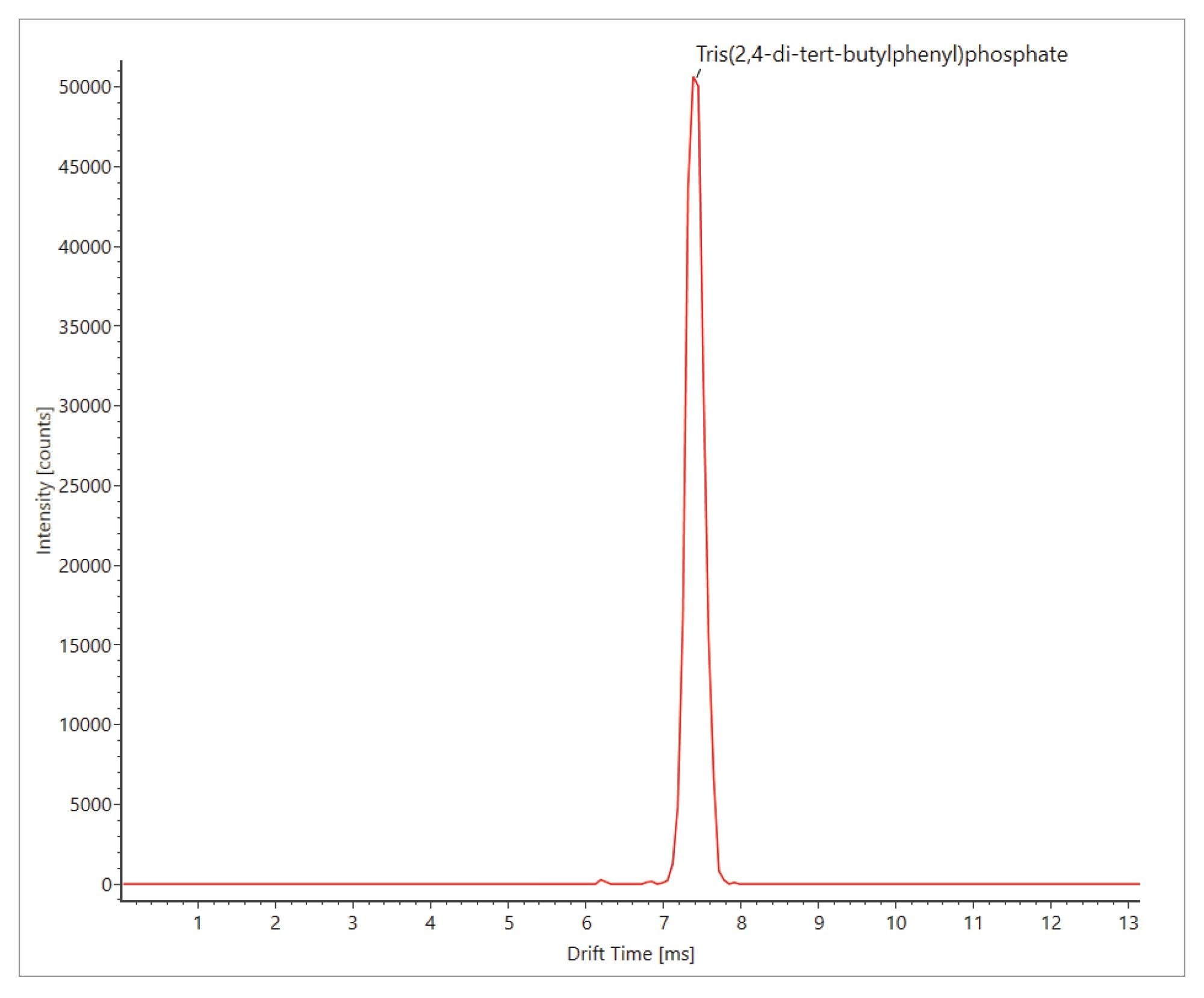
As can be seen from Figure 1, triphenylphosphine oxide, triphenyl phosphate, and tris(2,4-di-tert-butylphenyl) phosphate, have been detected where retention time error (<0.05 min,), mass accuracy (<3 ppm), expected product ion count (0–2) and Δ TWCCSN2< 1.65% have been observed. These analytes are ubiquitous plastic constituent contaminants, their identification in solvent and matrix blanks was achieved with confidence.24–25 Additionally, triacetin has been detected and identified with retention time error (<0.0 minutes), mass accuracy (0.9 ppm), expected product ion count (0) and Δ TWCCSN2 (0.5%). The results summary plot for triacetin (see Figure 1), reveal detections in the orange cordial food commodity, but not in blanks or blackcurrant with apple cordial. Triacetin is not indicated as a specific food additive on the food commodity labelling. Investigation of the commodity ingredients to rationalize detection of triacetin focused upon the ingredient listed as “Emulsifier glycerol esters of wood rosins”, which is used as an emulsifier in citrus-flavored soft drinks, preventing them from separating during distribution. Triacetin is a common food additive (E1518), used for instance as a solvent in flavorings, it has the formula C3H5(OCOCH3)3. It is classified as a triglyceride, i.e., the triester of glycerol. A natural product, glycerol ester of wood rosin is harvested from the stumps of longleaf (pinus palustris) and slash (pinus elliottii) pine tree and purified into a beverage-grade weighting agent. Triacetin may have been detected as natural constituent of wood rosin.
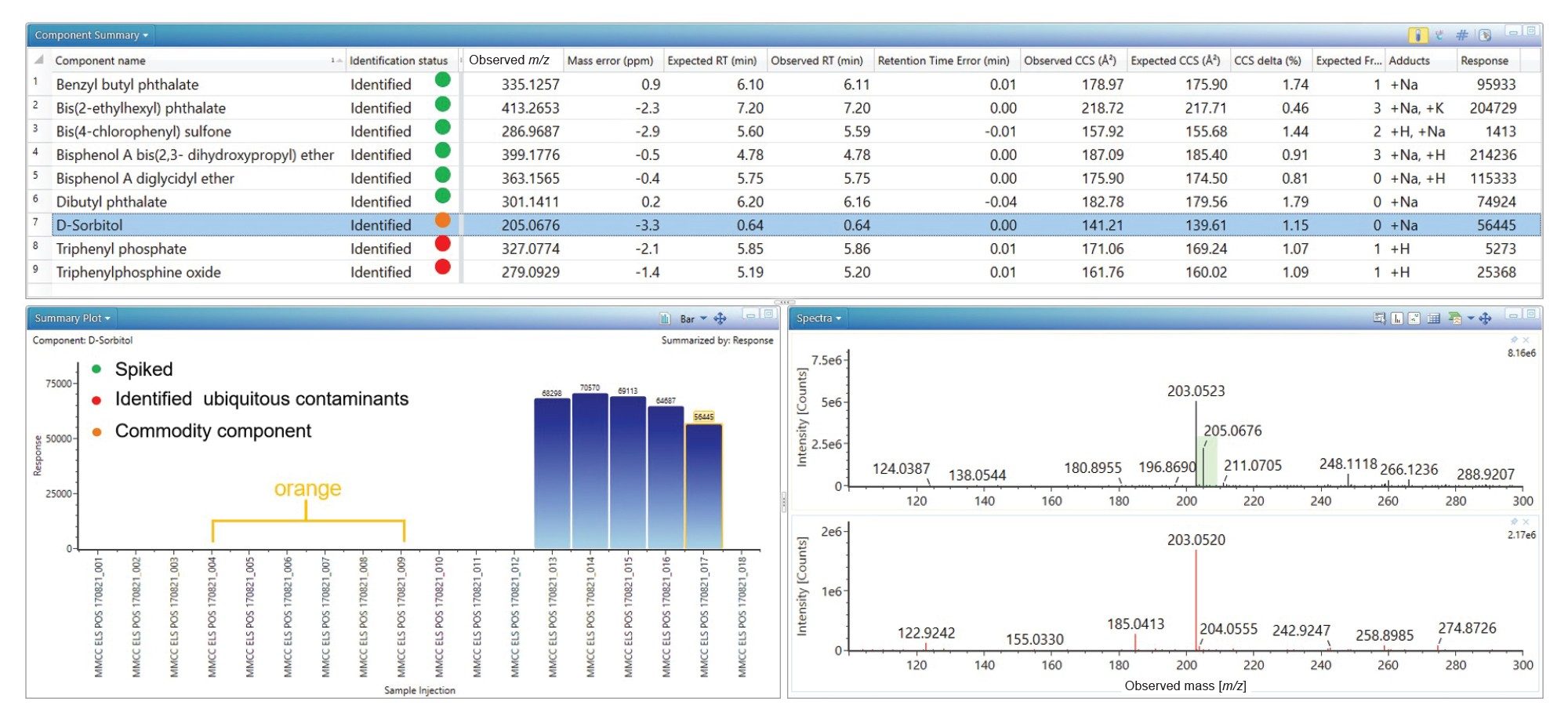
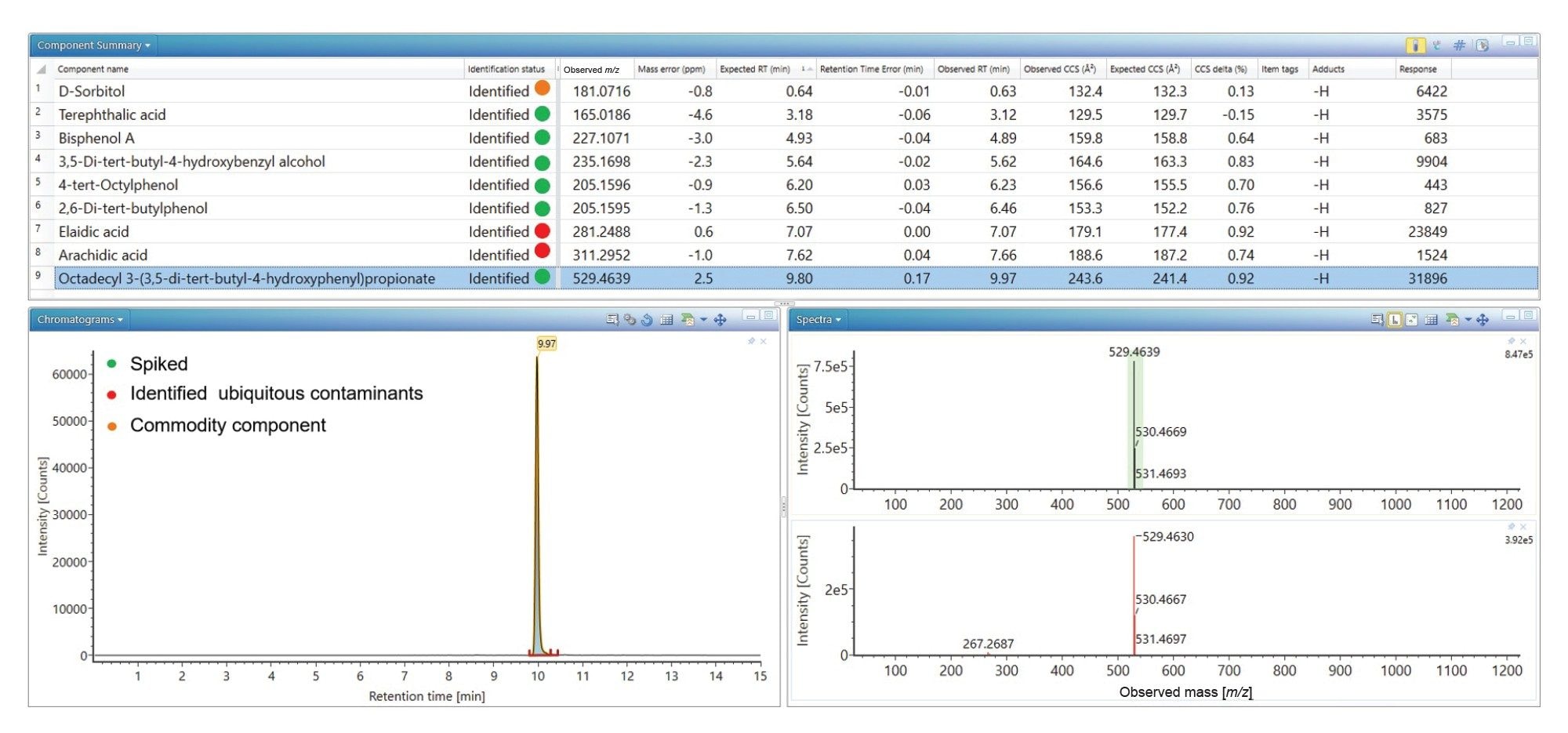
The spiked analytes were successfully identified in black currant with apple cordial, similarly ubiquitous plastic constituents were detected (see Figure 2). Additionally, D-sorbitol has been detected and identified with retention time error (0.0 minutes), mass accuracy (-3.3 ppm), expected product ion count (0) and Δ TWCCSN2 (1.15%). A CCS value has provided an additional descriptor in the absence of product ions. The results summary plot for D-sorbitol (see Figure 2), reveal detection in the black currant with apple cordial food commodity, but it is absent in orange cordial. On the food commodity labelling, D-sorbitol is not indicated as a specific food additive. The labelling indicates commodity constituents contain fruit juices from concentrate (Apple 9%, Blackcurrant 1%). As a natural product, D-sorbitol is found in both apple and black currants.26

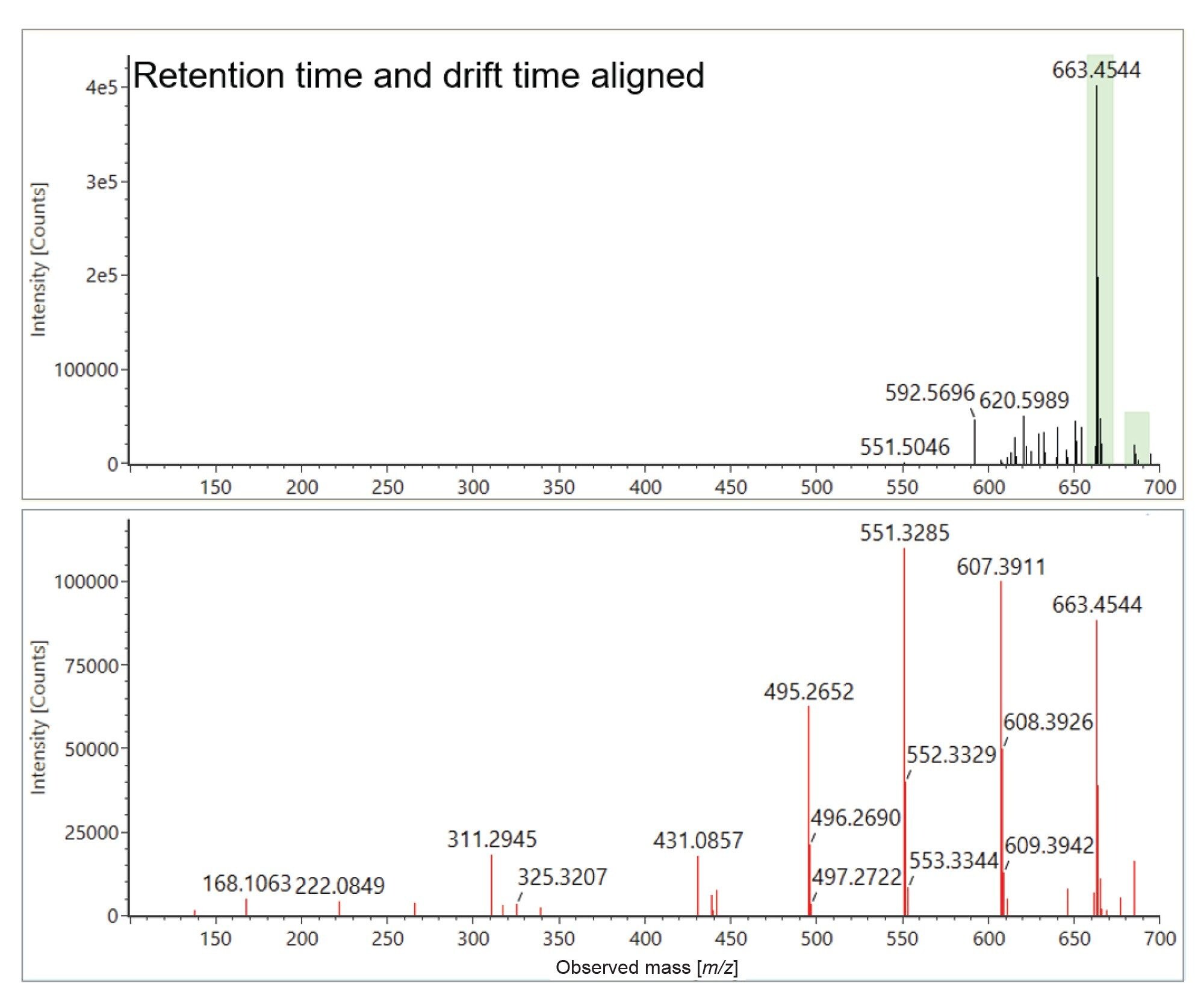
Verification was also performed using UPLC-IM-MS ES- analysis, with the E&L spiked analytes successfully identified (see Figure 4), where retention time error (<0.2 minutes), mass accuracy (<5 ppm), expected product ion count (0–3) and Δ TWCCSN2< 1% were observed. Dual polarity identification was achieved for D-sorbitol. Fatty acids (FAs), arachidic and elaidic acid, have also been identified. From the results summary plot, it was readily observed that a high background response was detected for elaidic acid, in solvent blanks and matrix blanks. Fatty acids have been reported as contaminants in solvents and may also leach from laboratory hardware/consumable items.27 However in orange cordial the response for elaidic acid is elevated, revealing that a component specific to orange cordial has been detected, indicating that a FA (C18) constituent has been identified. Fatty acid constituents of citrus fruits include oleic acid (C18:1), stearic acid (C18:0) and arachidic acid (C20:0).28–30 Further investigations using high purity standards were performed. For oleic acid, tr=7.01 minutes and average TWCCSN2=178.8 Å2 were determined. In the case of elaidic acid, tr=7.07 minutes and average TWCCSN2=179.8 Å2 were observed, confirming detection of elaidic acid. Orange cordial, commonly known as “orange squash”, is produced using orange comminute (squashed whole orange) providing a source of fatty acid food commodity content. It would be of interest to expand the E&L library FA content, to identify additional FA components evident in orange cordial and expand investigation of FA isomeric species utilising cyclic ion mobility mass spectrometry.
Conclusion
An ES+ and ES- UPLC-IM-MS library of E&L small molecules was generated comprising retention time tr, precursor ion, product ions and CCS values. This library can be used to facilitate non-targeted screening of E&Ls.
Verification of the library generated has been performed using a non-targeted screen of two food commodity samples. In the research performed identification of all spiked analytes provided confidence in reproducibility and the utility of ion mobility enhanced, mass spectrometry libraries. Additionally ubiquitous components of plastic were identified.
The diversity of the components of the E&Ls library and effectively the complexity of the challenge to screen for E&Ls, is illustrated by the detection of natural FA compounds, which are also “leachable” analytes, present in laboratory hardware and consumables. The sweetener food additive E 420 (D-sorbitol) identified in the black currant with apple cordial is a natural product present in apples and blackcurrants. Triacetin, also a food additive (E1518), identified in orange cordial, is probably a constituent of glycerol ester of wood rosins.
Ion mobility facilitates clean-up of precursor and product ion spectra by providing specificity in another dimension, in addition to CCS values. The quality, not just the size, of the library is of foremost importance for rapid determination of E&Ls in the samples.
For this research, performed using a UNIFI workflow, the specificity of tr, CCS, precursor and product ion library content enabled rapid identification and rationalization of observed analyte detections, illustrating the invaluable benefit of ion mobility mass spectrometry libraries to identify E&Ls.
References
- Food and Drug Administration, 2000, Code of Federal Regulation Chapter 21.
- Official Journal of the European Union, 2004, Regulation 1935/2004/EC.
- Official Journal of the European Union, 2006, Regulation 2023/2006/EC.
- Official Journal of the European Union, 2011, Regulation 10/2011/EU.
- Official Journal of the European Union, 2006, Regulation 1907/2006/EC.
- Official Journal of the European Union, 2009, Regulation 2009/49/EC.
- Official Journal of the European Union, 1987, Directive 87/357/EEC.
- Dzumana Z, Zachariasovaa M, Veprikovaa Z, Godulab M, Hajslovaa J. Multi-Analyte High Performance Liquid Chromatography Coupled to High Resolution Tandem Mass Spectrometry Method for Control of Pesticide Residues, Mycotoxins, and Pyrrolizidine Alkaloids. Anal. Chim. Acta. 2015; 863:29–40.
- Pérez-Ortega P, Lara-Ortega FJ, García-Reyes JF, Gilbert-López B, Trojanowicz M, Molina-Díaz, A. A Feasibility Study of UHPLC-HRMS Accurate-Mass Screening Methods for Multiclass Testing of Organic Contaminants in Food, 2016, Talanta 160, 704–712.
- Pérez-Ortega P, Lara-Ortega FJ, Gilbert-López B, Moreno-González D, García-Reyes JF, Molina-Día A. Screening of Over 600 Pesticides, Veterinary Drugs, Food-Packaging Contaminants, Mycotoxins, and Other Chemicals in Food by Ultra-High Performance Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry (UHPLC-Q-ToF-MS). Food Anal. Methods. 2017; 10:1216–1244.
- Romero-González R. Food safety: How Analytical Chemists Ensure It. Anal. Methods. 2015; 7:7193–7201.
- Coscollà C, León N, Pastor A, Yusà V. Combined Target and Post-run Target Strategy for a Comprehensive Analysis of Pesticides in Ambient Air Using Liquid Chromatography-Orbitrap High Resolution Mass Spectrometry. J. Chromatogr. A. 2014; 1368:132–142.
- Sjerps RMA, Vughs D, van Leerdam JA, ter Laak TL, van Wezel AP. Data-Driven Prioritization of Chemicals for Various Water Types Using Suspect Screening LC-HRMS. Water Research. 2016; 93:254–264.
- Pringle SD, Giles K, Wildgoose, J. An Investigation of the Mobility Separation of Some Peptide and Protein Ions Using a New Hybrid Quadrupole/Travelling Wave IMS/oa-ToF instrument. International Journal of Mass Spectrometry. 2014, 26, 1–12.
- Giles K, Pringle SD, Worthington KR, Little D, Wildgoose J, Bateman RH. Applications of a Travelling Wave-Based Radio-Frequency Only Stacked Ring Ion Guide. Rapid Commun. Mass Spectrom. 2004, 18, 2401.
- McCullagh M, Pereira C.AM, Yariwake JH. Use of Ion Mobility Mass Spectrometry to Enhance Cumulative Analytical Specificity and Separation to Profile 6‐C/8‐C glycosylflavone Critical Isomer Pairs and Known–Unknowns in Medicinal Plants. Phytochemical Analysis. 2019, 30(4), 1–13.
- McCullagh M, Douce D, Van Hoeck E, Goscinny S. Exploring the Complexity of Steviol Glycosides Analysis Using Ion Mobility Mass Spectrometry. Anal. Chem. 2018, 90, 4585–4595.
- McCullagh M, Pereira C.AM, Yariwake JH. Use of Ion Mobility Mass Spectrometry to Enhance Cumulative Analytical Specificity and Separation to Profile 6‐C/8‐C glycosylflavone Critical Isomer Pairs and Known–Unknowns in Medicinal Plants. Phytochemical Analysis. 2019, 30(4), 1–13.
- Goscinny S, McCullagh M, Far F, De Pauw E, Eppe G. Towards the Use of Ion Mobility Mass Spectrometry Derived Collision Cross Section as a Screening Approach for Unambiguous Identification of Targeted Pesticides in Food. Rapid Commun. Mass Spectrom. 2019, 33(S2), 1–15.
- Nye LC, Williams, JP, Munjoma NC, Letertre, MPM, Coen M, Bouwmeester R, Nicholson, JK, Plumb RS, McCullagh M, Gethings L, Lai S, Langridge J, Vissers JPC, Wilson ID. A Comparison of Collision Cross Section Values Obtained via Ion Mobility Spectrometry Following Direct Infusion and an Evaluation of U(H)PLC-IM-MS for the Characterisation of Metabolites in Rat Urine. J Chromatogr A 2019 Sep 27; 1602:386–396.
- McCullagh M, Goshawk J, Eatough D, Mortishire-Smith R, Pereira CAM, Yariwake JH, Vissers JPC. Profiling of the Known-Unknown Passiflora Variant Complement by Liquid Chromatography - Ion Mobility - Mass Spectrometry. Vissers. Talanta 221, 2021, 121311.
- Righetti L, Dreolin N, Celma A, McCullagh M, Barknowitz G, Sancho JV, Dall’Asta C. Travelling Wave Ion Mobility-Derived Collision Cross Section for Mycotoxins: Investigating Interlaboratory and Inter-Platform Reproducibility. J. Agric. Food Chem. 2020, 68, 39, 10937–10943.
- Goshawk J, Barknowitz G, and McCullagh M. A Workflow for Automatic MS Library Creation from Time-of-Flight Full-Spectra Data Processed in UNIFI. Waters, White Paper 720006783EN. 2020 Feb.
- Yang Y, Hu C, Zhong H, Chen X, Chen R, Yam KL. Effects of Ultraviolet (UV) on Degradation of Irgafos 168 and Migration of Its Degradation Products from Polypropylene Films. J Agric Food Chem. 2016 Oct 19;64(41):7866–7873.
- Hermabessiere L, Receveur J, Himber C, Mazurais D, Huvet A, Lagarde F, Lambert C, Paul-Pont I, Dehaut A, Jezequel R, Soudant P, Duflos G. An Irgafos® 168 story: When the Ubiquity of an Additive Prevents Studying Its Leaching From Plastics. Sci Total Environ. 2020 Dec 20; 749:141651.
- Jungmin L, Sorbitol, Rubus Fruit, and Misconception, Food Chemistry, Volume 166, 2015, Pages 616–622.
- Cheng YY, Yu JZ, Minimizing Contamination from Plastic Labware in the Quantification of C16 and C18 Fatty Acids in Filter Samples of Atmospheric Particulate Matter and Their Utility in Apportioning Cooking Source Contribution to Urban PM2.5. Atmosphere, October 2020 11(10):1120.
- Nordby H E, Nagy S. Fatty Acid Profiles of Orange and Tangor Juice Sac Lipids. Phytochemistry, 1971, Volume 10, Issue 3, Pages 615–619.
- Lamine M, Gargouri M, Rahali FZ and Mliki A. Authentication of Citrus Fruits Through a Comprehensive Fatty Acid Profiling and Health Lipid Indices: A Nutraceutical Perspective. Journal of Food Measurement and Characterization 2019 Vol. 13 Issue 3 Pages 2211–2217.
- Khan, FA, et al. "Comparative Evaluation of Physiochemical and Gc-MS Analysis of Sour Oranges and Sweet Oranges Peels Oil." Life Science Journal 2013;10(10s), 205–209.
720007655, June 2022