Method Development for the Chromatographic Separation of Synthetic Cyclic Peptides and Impurities Utilizing a Systematic Protocol and MaxPeak High Performance Surface Technology
Abstract
Waters has developed a systematic protocol, as outlined in the Waters MaxPeak™ Premier Reversed-phase Column Chromatography Kit.1 Following this protocol, methods were developed for a panel of cyclic peptides using the Arc™ Premier System and Premier columns featuring MaxPeak High Performance Surface (HPS) technology. Using this advanced technology, we were able to demonstrate improved separations when compared to traditional stainless-steel systems and columns. The resulting method was able to obtain acceptable performance for the separation of the five cyclic antibiotic peptides oritavancin, dalbavancin, caspofungin, daptomycin, and anidulafungin. Further, a method was created to separate dalbavancin from its associated impurities.
Benefits
- The use of a systematic protocol reduced method development time
- Improved chromatographic peak performance and reproducibility was observed for cyclic peptides using the Arc Premier System and columns featuring MaxPeak HPS technology
- The reliable and adaptable Arc Premier System featuring a column manager in combination with the QDa™ Mass Detector allowed larger DOE experiments and easier peak identification
Introduction
Cyclic peptides are of particular interest due to their unique properties. Their lack of a free N- or C- terminus reduces their proteolytic degradation thus increasing their half-life in the human body allowing for less frequent dosing when used as a treatment.2 The reduced conformational freedom of these compounds also increases the binding affinity and specificity when compared to linear peptides.3 Further benefits of cyclic peptides over linear peptides is their ability to form intramolecular hydrogen bonds, reducing the polarity and allowing them to traverse cellular lipid bilayers easier.
Antibiotics have saved countless lives since they were discovered, but frequent prescription of these compounds has led to resistant strains of bacteria becoming more prevalent. In some cases, cyclic peptide antibiotics have been shown to be successful in combatting specific and resistant strains. Daptomycin and dalbavancin have been shown to be effective treatments against methicillin-resistant staphylococcus (MRSA).4 MRSA has become more prevalent in recent years, increasing from 21% in 2016 to 35% in 2020 of bloodstream infections worldwide.5 Recently, new strains of bacteria showing resistance to these cyclic peptides are emerging. With growing interest in these peptides, there is a need for faster more streamlined methods.
Analytical Quality by Design (AQbD) has become a topic of interest for regulatory agencies and the pharmaceutical industry over recent years, and the principles of AQbD are now being adopted into regulatory guidance. One approach to improve method understanding and assess the potential impact of analytical procedure parameters on the analysis is to use a standardized, systematic approach to developing a method. Recently Waters has released a systematic protocol for the chromatographic method development of peptide applications. Based on prior knowledge, method screening, and knowledge gained through use of the systematic approach, a risk assessment was conducted to ensure confidence in the quality and consistency of the final method.
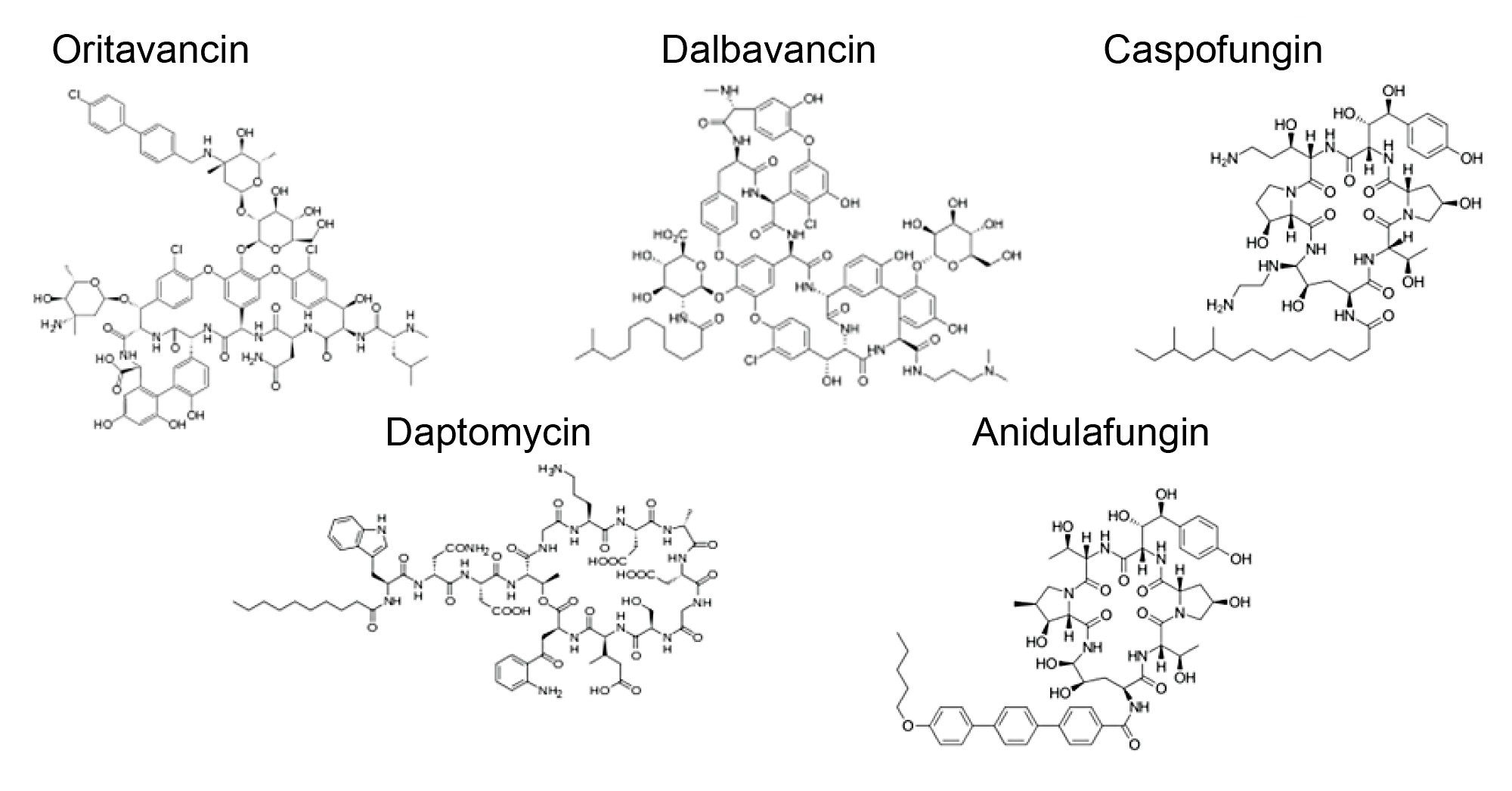
In this application note, we show the utilization of this systematic method development approach for the creation of a method to separate the five cyclic antibiotic peptides shown in Figure 1. Following a successful method development for the panel of cyclic peptides, a method was created for separating the impurities of dalbavancin.
Experimental
Sample Preparation and Materials
Individual peptide standards were obtained from Cayman Chemical (MI, USA); dalbavancin impurity A40926 was purchased from Toronto Research Chemicals (ON, Canada). Standards were prepared in 100% dimethyl sulfoxide (DMSO) and samples were prepared in 100% DMSO at 0.1 mg/mL of oritavancin, dalbavancin, caspofungin, daptomycin, and anidulafungin each. Impurity samples contained 0.1 mg/mL of dalbavancin and 0.2 mg/mL of A40926 prepared in 100% DMSO.
LC Conditions
|
LC system: |
Arc Premier QSM-r |
|
Detection: |
PDA 2998 @ 214nm |
|
Vials: |
1mL Total Recovery Vial, 186000385DV |
|
Column(s): |
XSelect™ Premier Peptide CSH C18 130 Å, 2.5 µm, 4.6 mm X 150 mm Column |
|
Column temperature: |
Peptide Panel: 60°C, Dalbavancin Impurities: 80°C |
|
Sample temperature: |
20 °C |
|
Injection volume: |
10 μL |
|
Flow rate: |
0.96 mL/min |
|
Mobile phase A: |
0.1% Formic Acid in Water |
|
Mobile phase B: |
0.1% Formic Acid in Acetonitrile |
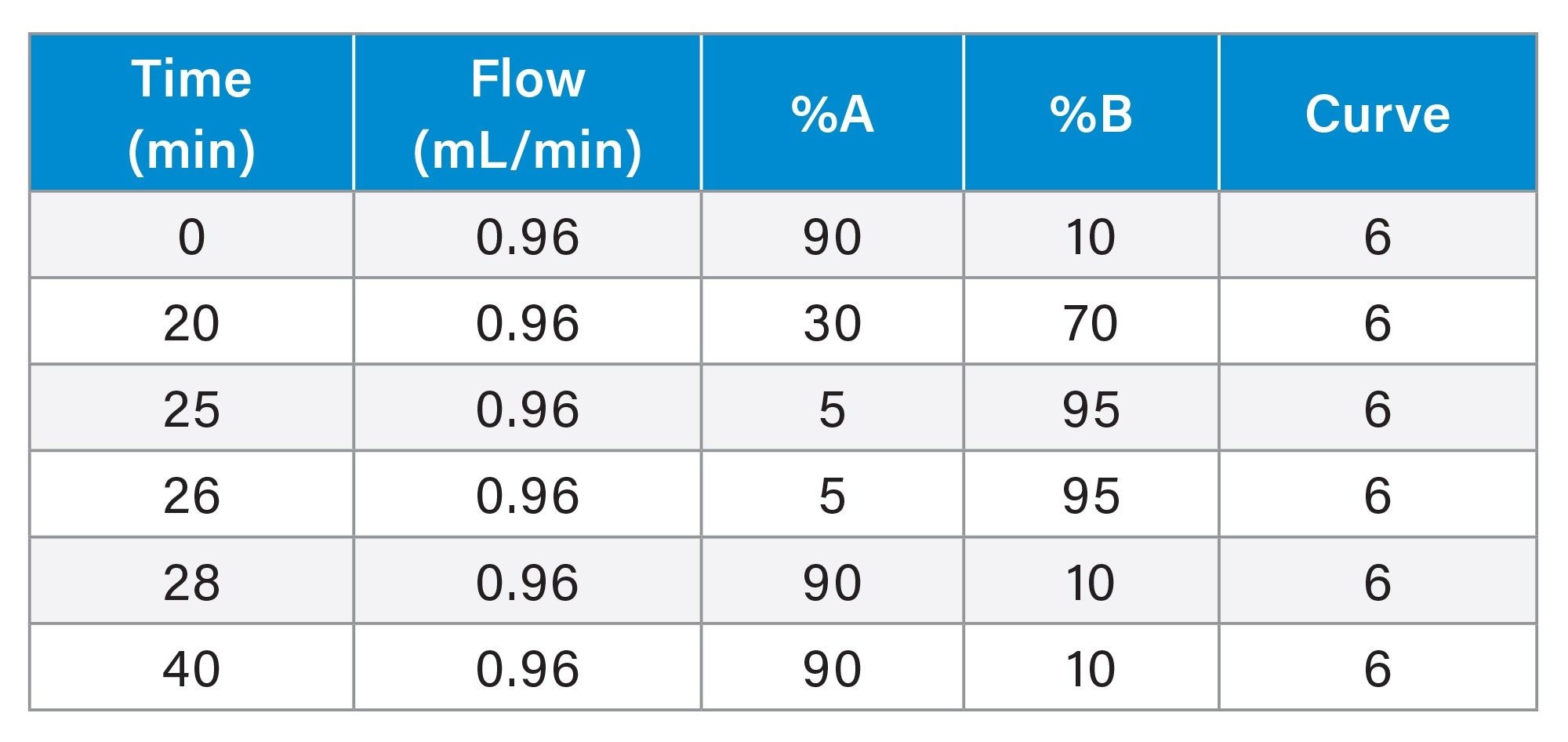
Panel Gradient
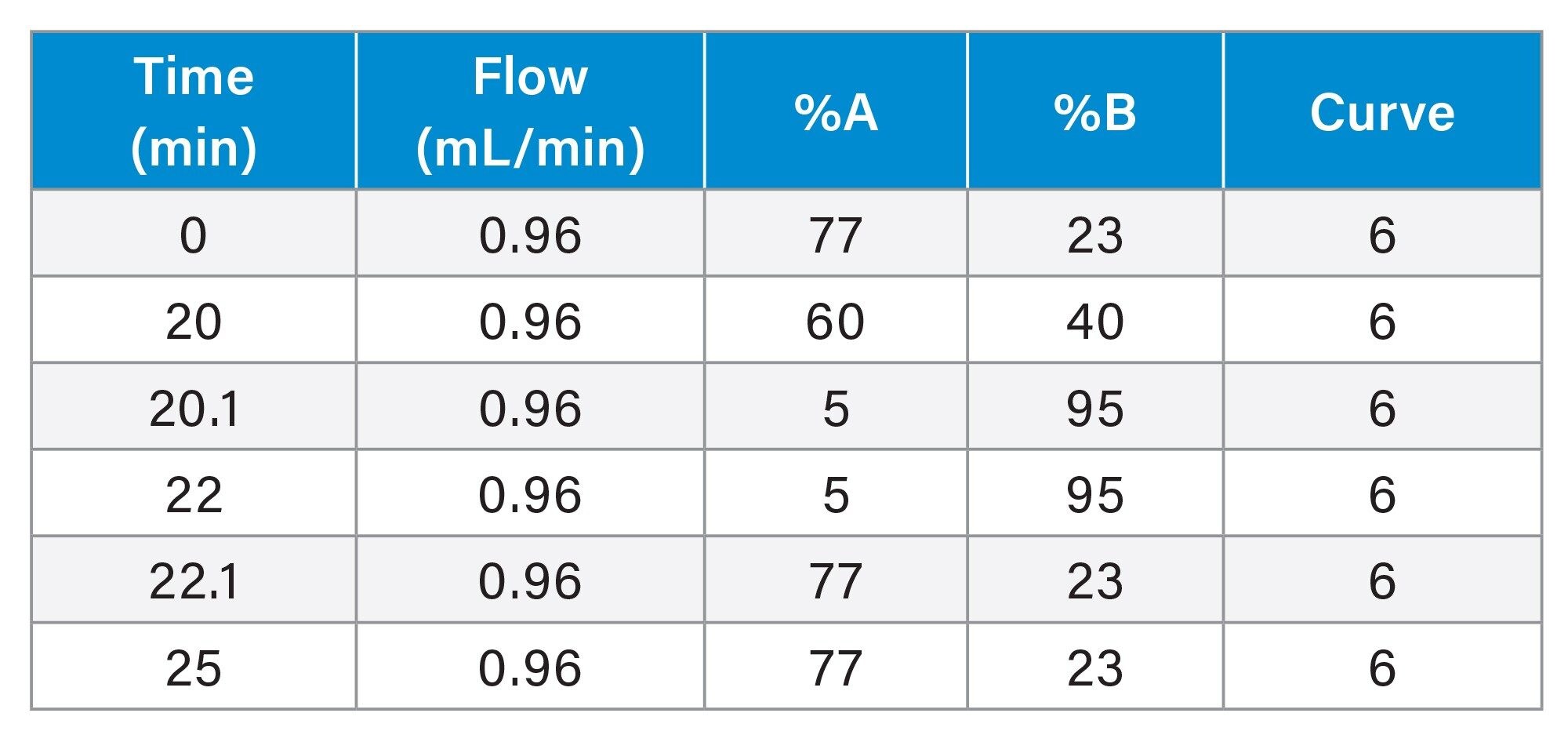
Dalbavancin Impurity Gradient
Data Management
|
Chromatography software: |
Empower 3.6.2 |
Results and Discussion
The systematic protocol and supporting studies facilitated an understanding of the various risks associated with the analysis of peptides. The most important considerations in the protocol are as follows:
- Utilization of the XSelect Premier Peptide CSH C18 (XSelect) Column and the XBridge Premier Peptide BEH C18 (XBridge) Column.
- Use of 0.1% formic acid followed by 0.1% trifluoracetic acid (TFA) as additives in both the aqueous and acetonitrile mobile phases.
- A screening gradient from 0.5% Acetonitrile (ACN) to 55% ACN in 20 minutes.
- A focus gradient is created based on the elution time of analytes in the screening gradient.
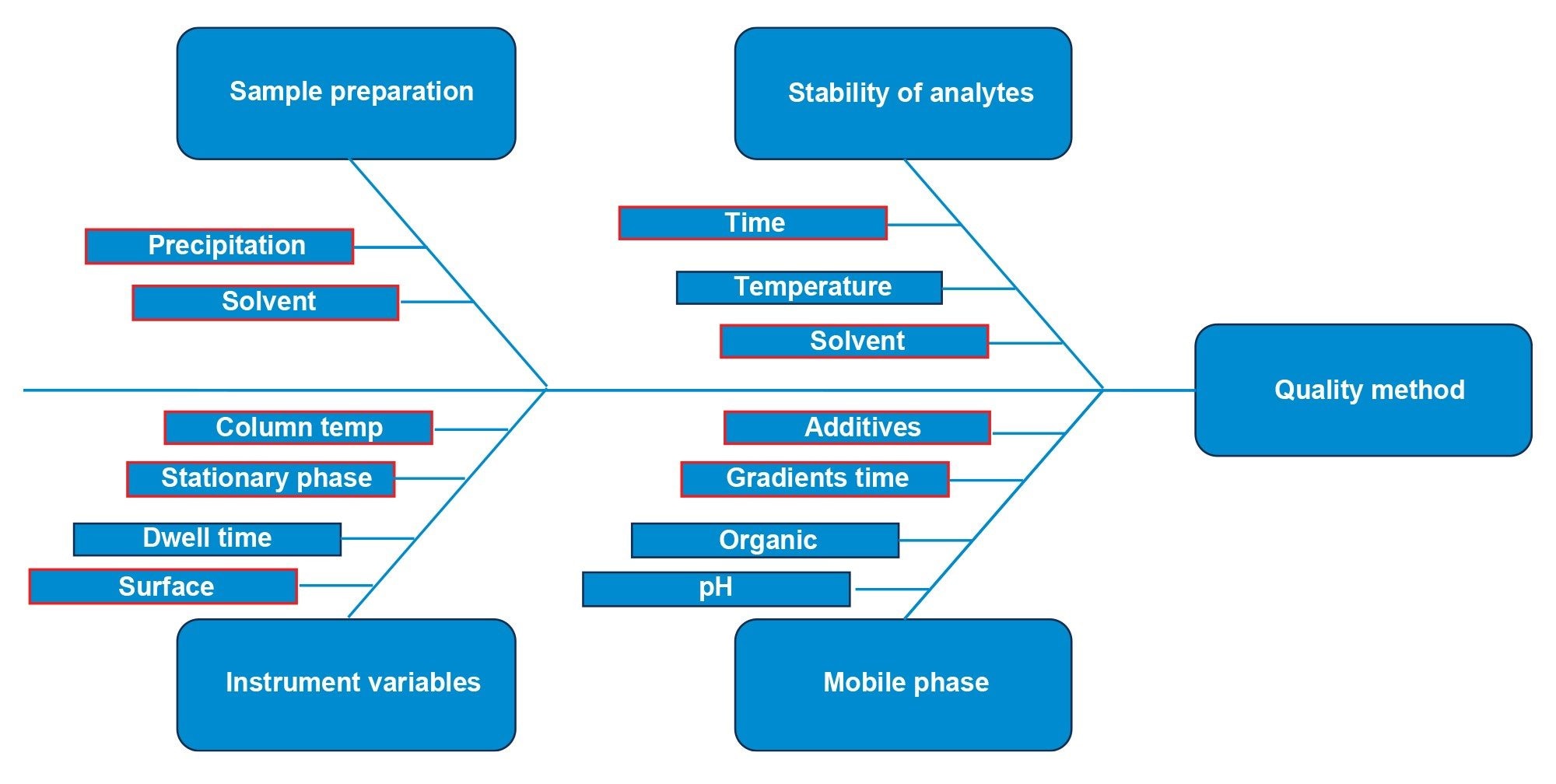
The four column and eluent combinations provide differences in selectivity and retentivity along with differences in chromatographic peak shape. During literature review and initial testing, variables were identified as higher and lower risk. The fishbone diagram in Figure 2 outlines the variables identified.
Sample Preparation and Analyte Stability
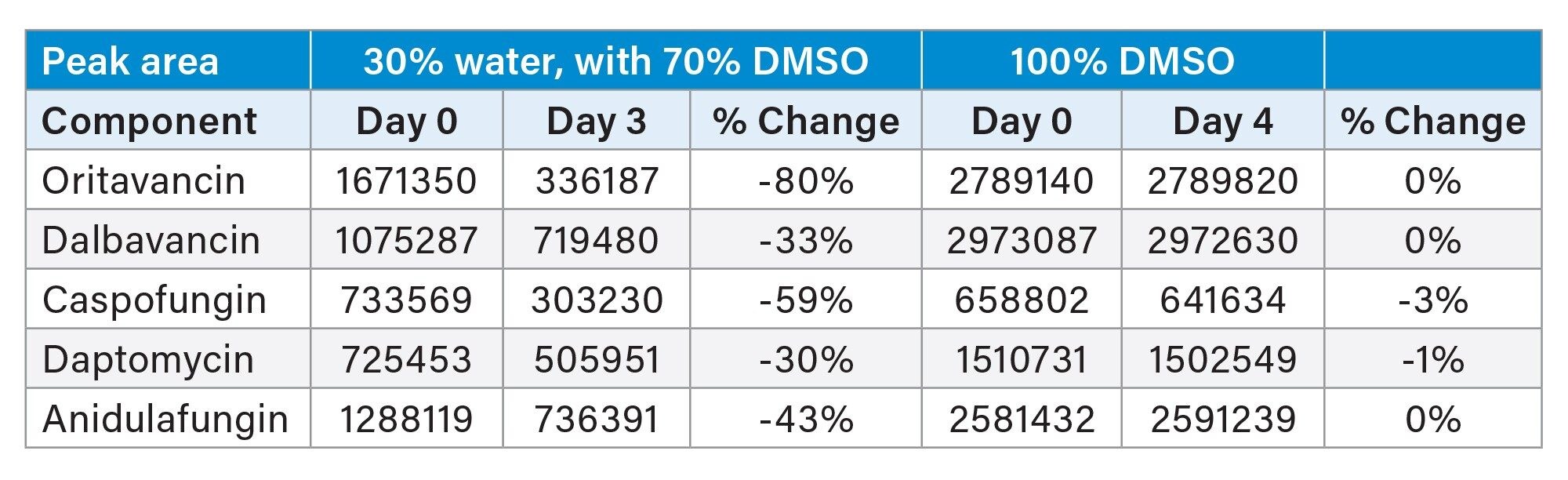
The first high risk variables encountered during the method development process were with sample preparation. Solubilizing the samples, precipitation, and sample stability proved to be a challenge. Stock solutions of 1 mg/ml of each peptide were made in 100% DMSO and further diluted. Notably the highest aqueous content that could solvate the samples at 0.1 mg/ml was 30% water, with 70% DMSO. This 30% aqueous solvent proved to have issues as rapid degradation was observed (Table 1.) This observation led to changes in sample preparation. Lower aqueous amounts were tested but all showed similar results. In the end a 100% DMSO sample preparation was found to work the best. This introduced the possibility of strong solvent effect, but no peak splitting was seen in the final method.
Instrument Variables and Mobile Phases
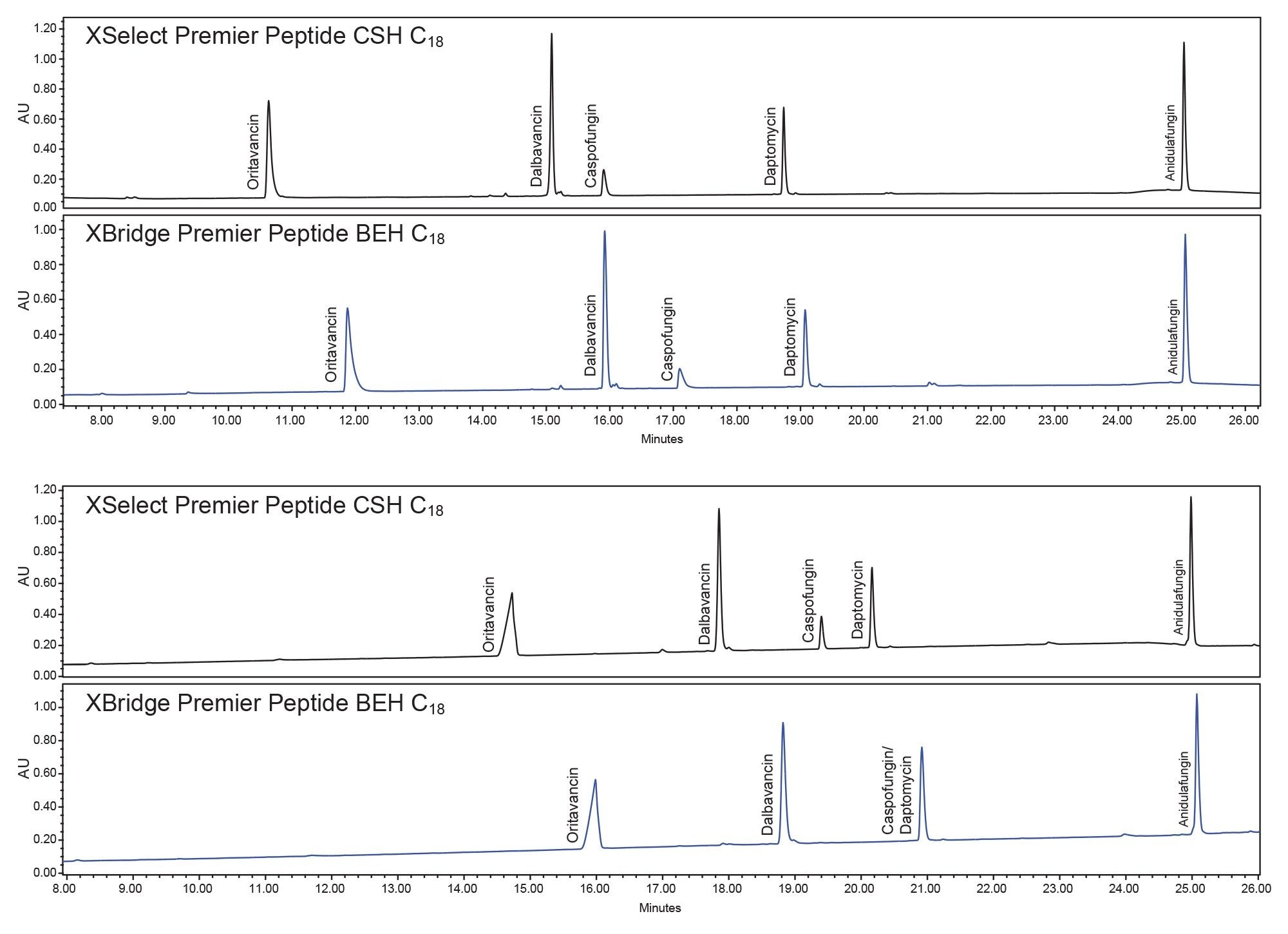
While investigating the sample stability issues, the four different column and eluent additive combinations were tested with freshly prepped samples. The results can be seen in Figure 3; notably the XSelect Column provides the best separations. Differences in selectivity can be seen in the bottom chromatogram with the XBridge Column and the TFA mobile phase additives which led to a coelution between daptomycin and caspofungin. The QDa Mass Detector allowed easy identification of the peaks and allowed for the confirmation of the coelution.
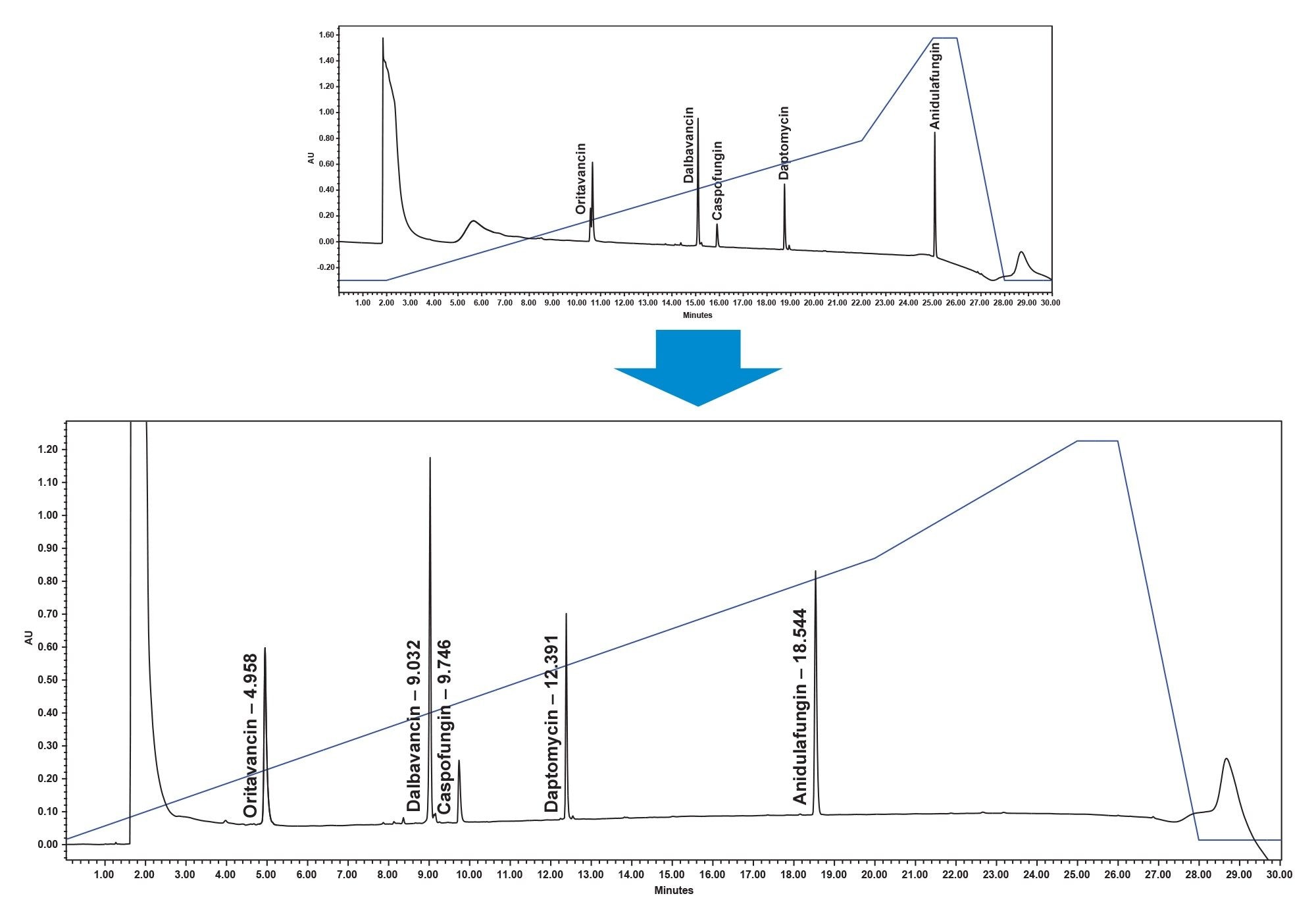
While screening these four combinations the chromatographic peaks were noticed to elute late in the gradient. The first chromatographic peak, oritavancin, is shown to elute more than 10 minutes into the run and anidulafungin, the last eluting peak, elutes during the slow ramp to wash. Therefore, the initial starting point was shifted from 0.5% ACNC to 10% ACN and the end point was changed from 55% ACN to 70% ACN. This shift to a 10% ACN starting point also meant that the strong solvent effect could be eliminated when compared to the 0.5% ACN starting point. With this change a 100% DMSO sample preparation became viable. The final method for the panel separation can be seen in Figure 4. This new method allows the first peak to elute earlier in the gradient and the last peak to elute in the main gradient, as well as improve the chromatographic peak characteristics.
Risk Reduction of MaxPeak Technology
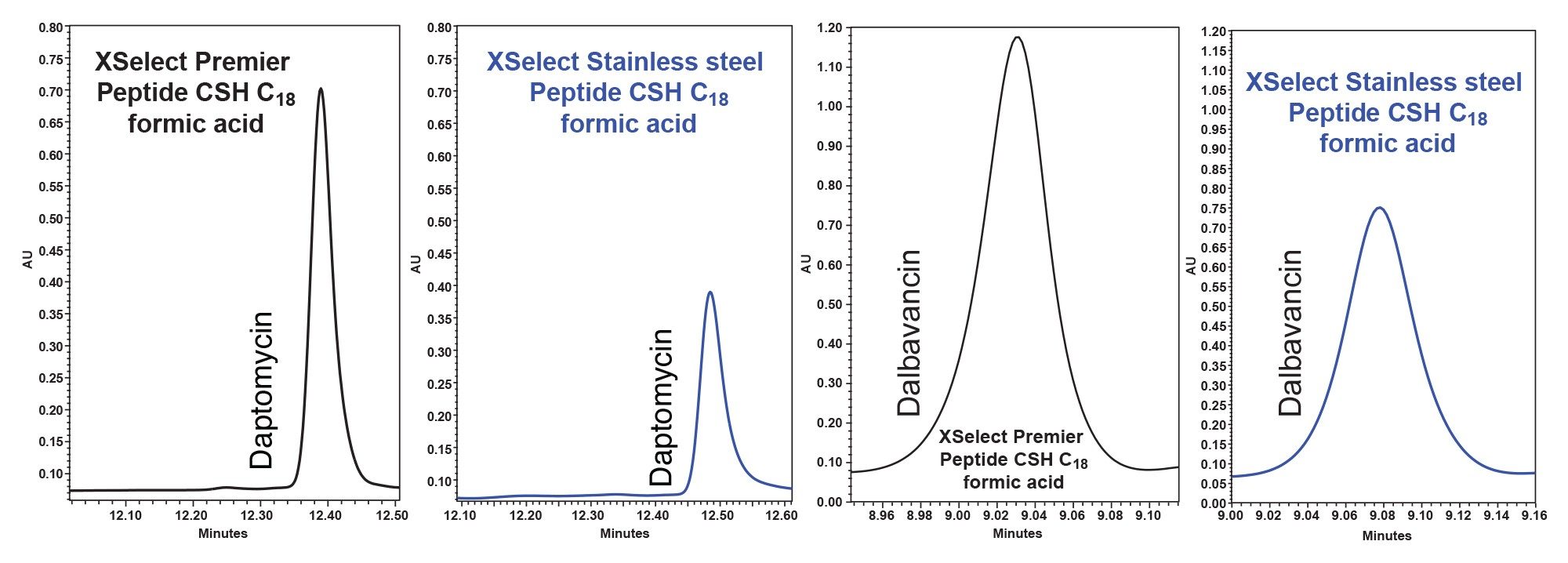
After these method modifications were made, they were run on two systems, the Arc Premier featuring the MaxPeak HPS technology and the ACQUITY Arc featuring traditional stainless-steel hardware. The comparison of these two hardware surfaces was of particular interest as these compounds can be Lewis bases which can adsorb onto the metal oxides on the stainless-steel surfaces. This adsorption can cause degraded chromatographic performance as is seen in Figure 5. Substantially reduced peak height, shape, and area was observed on the stainless-steel system when compared to the Arc Premier System.
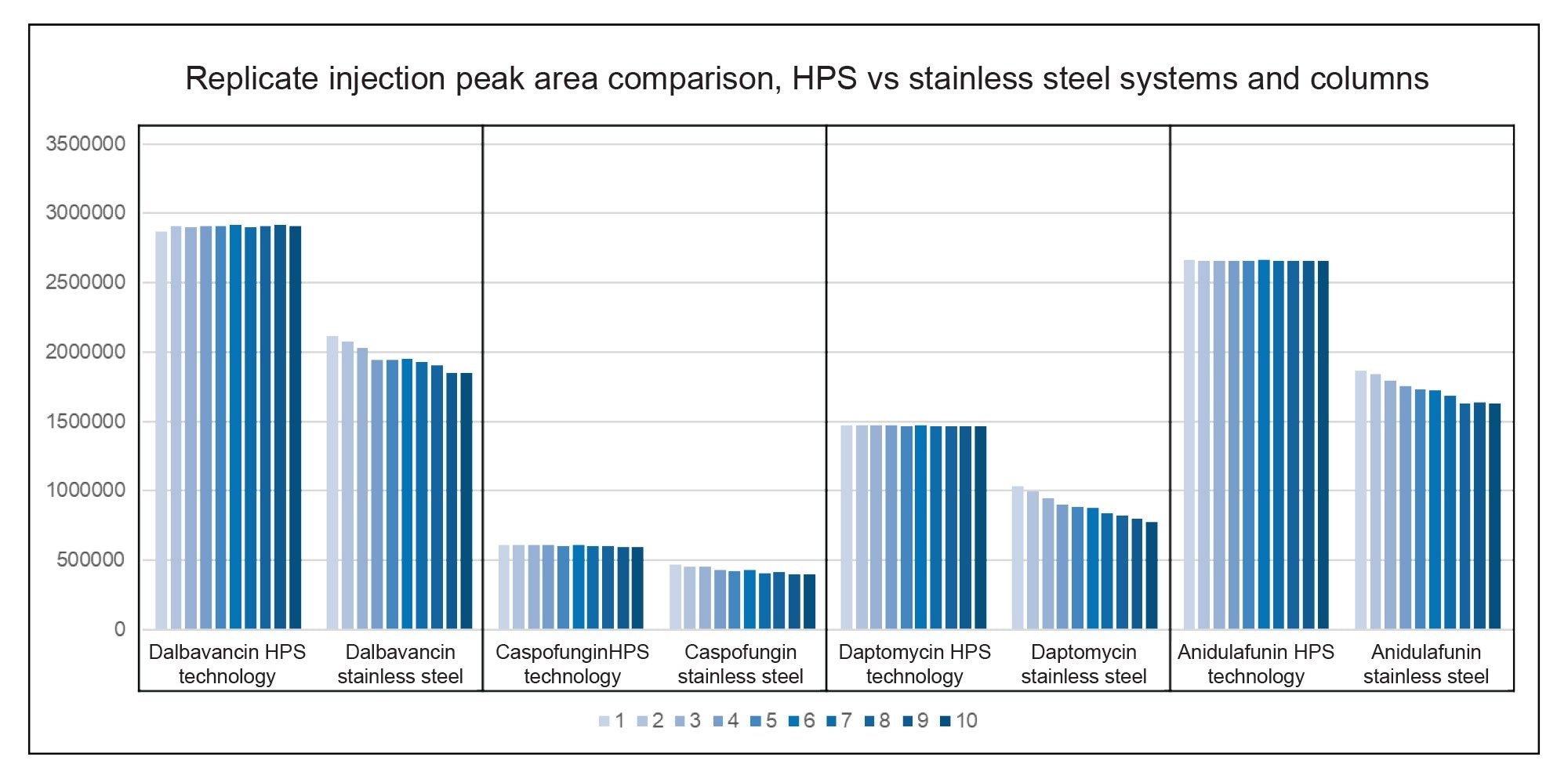
The following chart in Figure 6 shows the results of ten replicate injections run on the Arc Premier and ACQUITY Arc systems. These graphs provide a visual of the relative standard deviations of these runs. The traditional stainless-steel systems are less reproducible when compared to systems and columns featuring MaxPeak HPS technology. Note that these runs are done on non-conditioned systems and columns. The reproducibility could likely be improved on the stainless-steel system if it was conditioned with particular mobile phases and had sacrificial analyte injections to adsorb onto all active sites. With MaxPeak HPS, no such process is required which reduces risk, saves time, and increases the number of useful injections.
Dalbavancin Impurity Analysis
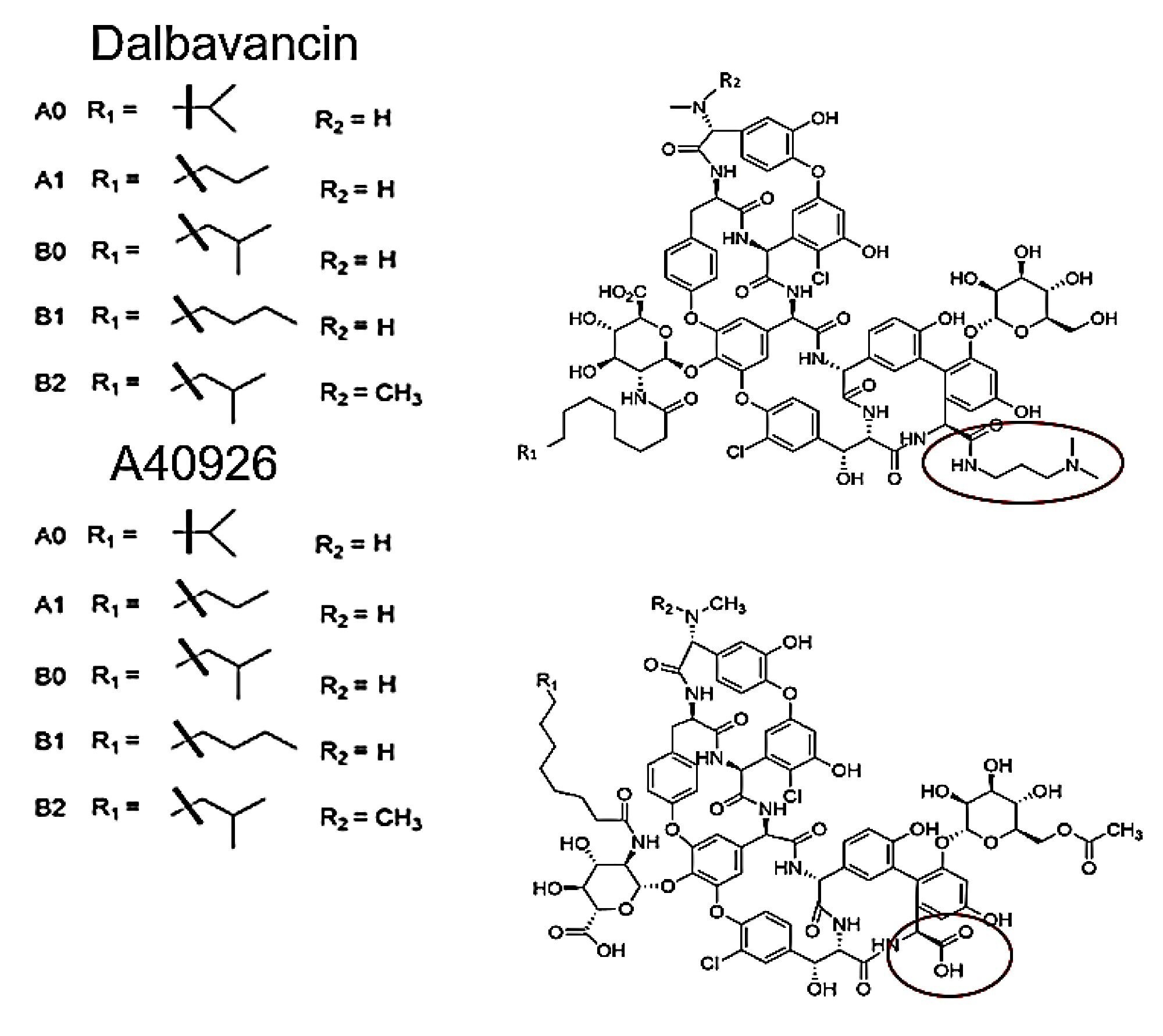
Following the analysis of the panel of cyclic peptides, a method was created for the impurity analysis of dalbavancin which is an antibiotic active against gram positive bacteria. Dalbavancin is a teicoplanin analog and a dimethylaminepropyl amide derivative of the peptide A40926.6 Both Dalbavancin and A40926 are comprised of the main compound B0 but also contain small amounts of A0, A1, B1, B2 these known impurities are allowed in drug products but must still be monitored. An impurity panel was created using these 10 compounds, a method was then created to separate all impurities from the main component dalbavancin B0. The structure of these 10 compounds can be seen in Figure 7.
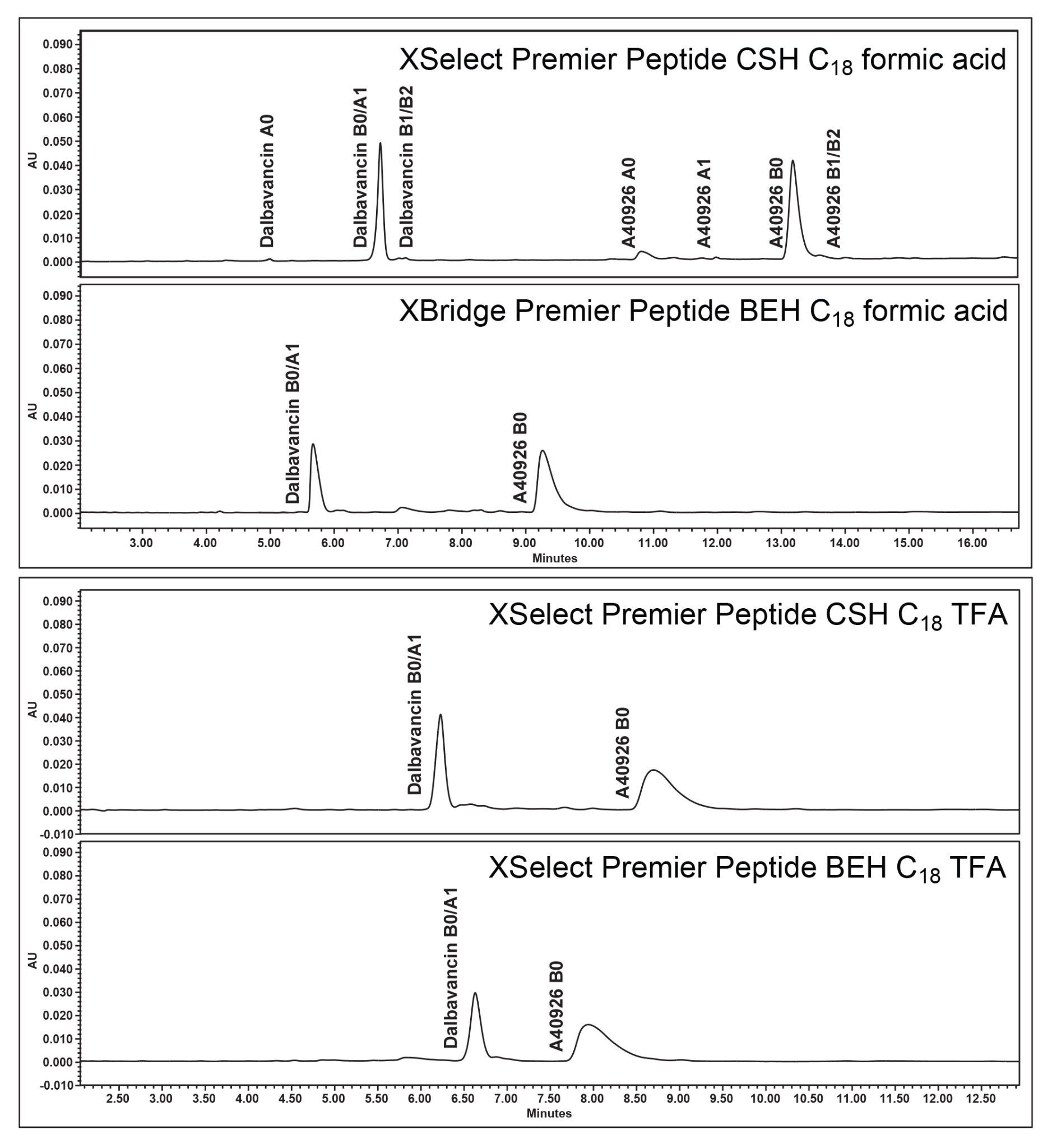
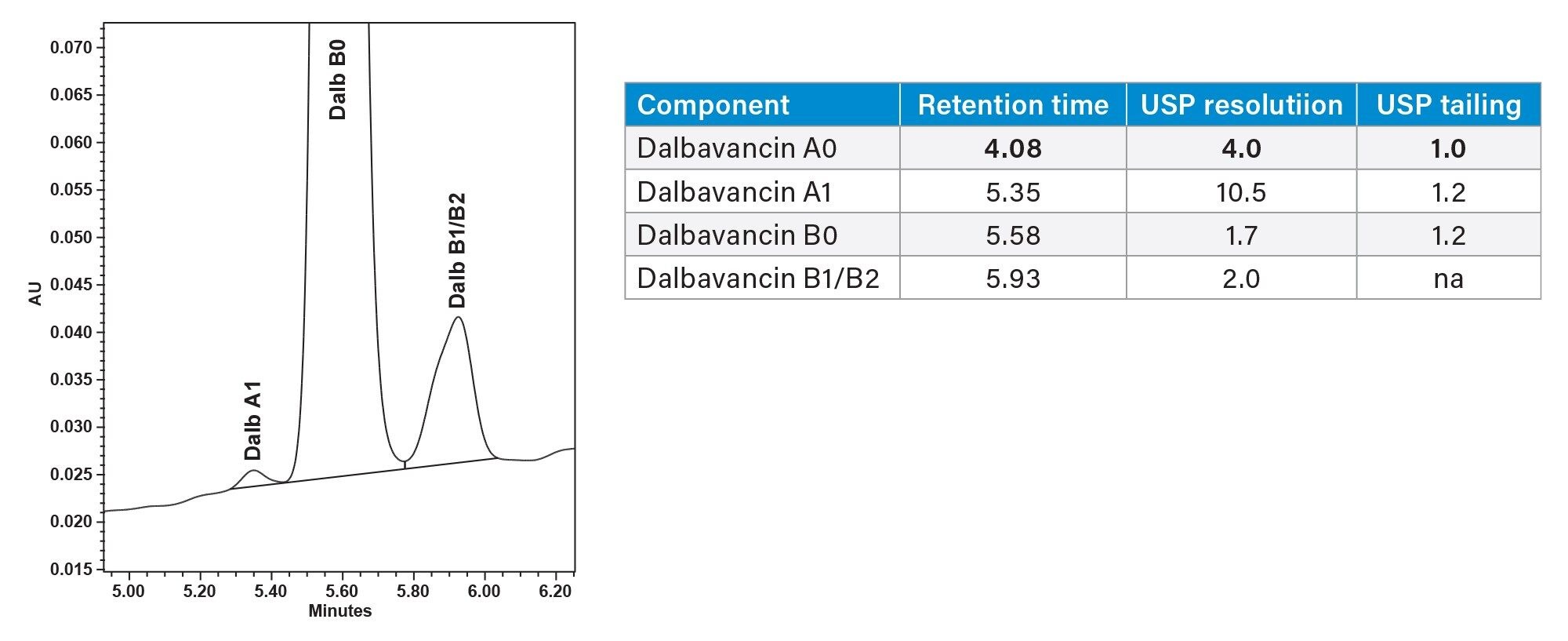
In Figure 8 each combination of column and mobile phase additive had specific gradients created to allow them the best chance of separations and to get approximately the same retention times in the chromatograms. The XSelect Column with formic acid eluent additive was found to perform the best, using the QDa Mass Detector all 10 compounds were identified in this chromatogram. The other combinations did not provide separation where all compounds were identifiable.
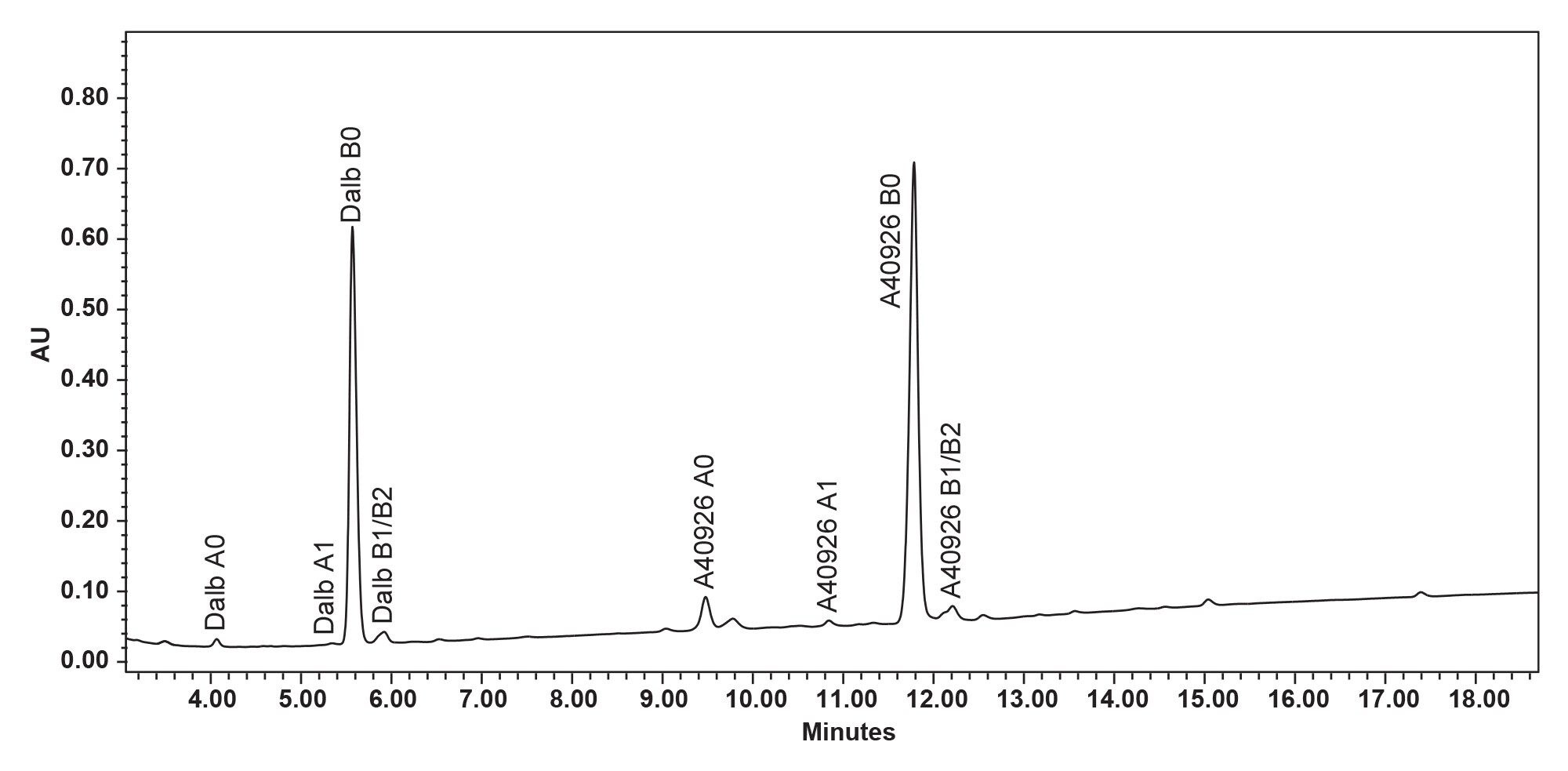
While the XSelect Column is the best performing in the screening runs in Figure 8 it still cannot sufficiently resolve dalbavancin A1 from dalbavancin B0. In order to resolve the dalbavancin B0 from its associated impurities including dalbavancin A1, both gradient time and temperature was further optimized on all 4 columns. Again, XSelect was found to be the best at providing the desired separation. The final method parameters included an 80 °C column temperature and a gradient seen in the gradient table at the beginning of this application note (page 3). Dalbavancin B0 was completely separated from the other 9 compounds. (Figure 9, 10).
Conclusion
The systematic protocol as outlined in the Waters MaxPeak Premier Reversed-phase Column Chromatography Kit has proven to be an excellent starting point for creating methods. The MaxPeak HPS technology featured in premier systems and columns provided increases of up to 62% in peak area, 67% increase in peak height, 35% reduction in peak tailing, and up to 9% less relative standard deviation of chromatographic peak area in replicate injection when compared to traditional stainless-steel systems and columns.
Following the systematic protocol, a HPLC method was successfully created on the Arc Premier System to separate a panel of 5 cyclic antibiotic peptides including oritavancin, dalbavancin, caspofungin, daptomycin, and anidulafungin. To further demonstrate the ability of the systematic protocol an impurity analysis was conducted with a slightly expanded design of experiment criteria, this method was able to separate all 9 impurities from the compound of interest dalbavancin B0. The QDa Mass Detector was a useful tool to identify the potential impurities found in dalbavancin by mass to charge ratio.
References
- MaxPeak™ Premier Peptide Reversed-Phase Columns and Method Screening Kit: Practical Steps in Developing Robust Peptide Separations Start Up Guide. Waters Corporation. User Manuals, 720008131, 2023.
- Van Bambeke F. Lipoglycopeptide Antibacterial Agents in Gram-Positive Infections: A Comparative Review. Drugs 2015;75: 2073–2095. DOI: 10.1007/s40265-015-0505-8
- Xiao P, Pei D. High-Throughput Synthesis and Screening of Cyclic Peptide Antibiotics. Journal of Medicinal Chemistry 2007; 50(13): 3132–3137. DOI: 10.1021/jm070282e
- Werth BJ, Jain R, Hahn A, Cummings L, Weaver T, Waalkes A, Sengupta D, Salipante SJ, Rakita RM, Butler-Wu SM. Emergence of Dalbavancin Non-Susceptible, Vancomycin-Intermediate Staphylococcus Aureus (VISA) After Treatment of MRSA Central Line-Associated Bloodstream Infection with a Dalbavancin- and Vancomycin-Containing Regimen. Clin Microbiol Infect. 2018 Apr;24(4):429. doi: 10.1016/j.cmi.2017.07.028.
- World Health Organization. World Health Statistics 2023. World Health Organization, 19 May 2023, www.who.int/publications/i/item/9789240074323.
- N. Scheinfeld. Dalbavancin: A Review for Dermatologists. Dermatology Online Journal, 2006 12(4). DOI: 10.5070/D30wn7d4q9
720008273, March 2024