Improving the Purification Workflow With MaxPeak™ Premier OBD™ Preparative Columns: Isolation of Compounds From a Vitamin Beverage
Abstract
Whether target compounds for isolation are well-characterized or impurities with unidentified properties, the purification scientist requires a reliable column which provides good peak shape and sensitivity. Some compounds introduce another uncertainty for the prep chromatographer – non-specific adsorption to metal oxide surfaces. Stainless steel chromatographic columns and prep HPLC system components are prone to these unwanted interactions and can cause reduced peak sensitivity, poor peak shape, or limited peak detection, which impact fraction triggering and compound recovery. Previous work discussed the enhancement of LC-MS/MS analysis of B-group vitamins using MaxPeak high performance surfaces technology.1 The methodology employed was modified in these experiments to illustrate how MaxPeak Premier OBD Preparative Columns were used to isolate four B vitamins and two unknowns in a preparative workflow. The purification workflow presented provides a model that mirrors the strategies that might be used by the core support scientist who isolates many different types of compounds or the process development chemist performing impurity isolation. A commercially available vitamin beverage was used for all the experiments performed in this study. MaxPeak Premier OBD Preparative Columns are manufactured with Waters’ Hybrid Particle2,3 and Optimum Bed Density [OBD]4 Technologies for consistent column-to-column performance, long column lifetimes, and direct scalability from analytical to prep, as well as inert surfaces to mitigate the undesirable effects of compound to metal surface interactions.
Benefits
- Reduce unwanted interactions between certain compounds and the stainless steel (or other metal alloys) components in the column, promoting enhanced target compound detection, improved peak shape for precise fraction triggering, and leading to improved compound isolation and enhanced peak sensitivity for greater confidence in detecting impurities at low levels in crude sample mixtures
- Provide full scalability from UPLC™ to prep for predictable target collection using Waters’ highly-controlled OBD column packing process, ensuring that preparative columns are of similar bed density to analytical columns of the same chemistry
- Save time by eliminating column conditioning to reduce non-specific adsorption prior to starting purification
Introduction
The isolation of compounds is simplified in LC purification if the sample components are resolved with good peak shape in detectable quantities and adequate signal to noise ratio. Although the laboratory scientist has many tools at their disposal for optimizing separations and isolating compounds, there are times when broad and tailing peaks, low sensitivity or undetected targets complicate the purification workflow. These undesirable outcomes lead to poor fraction triggering and collection, and most probably, low target purity and sample loss.
High mechanical strength, extensive compatibility with chemicals, and ease of fabrication make stainless steel an attractive material for the manufacture of high-performance liquid chromatography columns and instruments used in a wide variety of industrial applications. Despite these favorable characteristics, stainless steel can negatively impact the peak shape and recovery of some compounds.5 Negatively charged molecules can interact ionically with the positively charged metal oxide surfaces of the column and LC flow path in neutral and acidic pH environments and lead to non-specific adsorption.6 This non-specific adsorption (NSA) occurs with stainless steel and titanium, as well as other metal alloys. Metal sensitive compounds with strong acidic moieties like sulfates, phosphates, or carboxylic acids are prone to adsorption. Classes of compounds that may adsorb to metal surfaces include organic acids, organophosphates, phosphopeptides, acidic peptides, acidic glycans, oligonucleotides, acidic phospholipids, nucleic acids, and other metal chelating compounds.
Most purification workflows start with an analytical assessment of the sample mixture on one or more different column chemistries at both low and/or high pH, and the column that delivers the best result is chosen for the subsequent compound isolation. For those laboratories isolating compounds from synthetic compound libraries, the large number of samples are batched and must be purified efficiently to save both precious sample as well as process time. Such samples require protocols that will predictably provide the best purification outcome in one prep injection. When pH switching is employed in the purification scheme on a stainless-steel column, the high pH mitigates adsorption. Therefore, switching between low and high pH deems the first injection after a high pH separation susceptible to non-specific adsorption once again. While there are several other solutions for dealing with the non-specific adsorption issue, including passivation of surfaces with acid, conditioning surfaces with sample or matrix, using PEEK or PEEK lined steel columns, or using additives in the mobile phase, all have their unique drawbacks and are time consuming. MaxPeak Premier OBD Preparative Columns are designed to minimize compound/surface interactions that can lead to unsatisfactory purification outcomes, no matter which pH is used for isolation.
Experimental
Sample Description
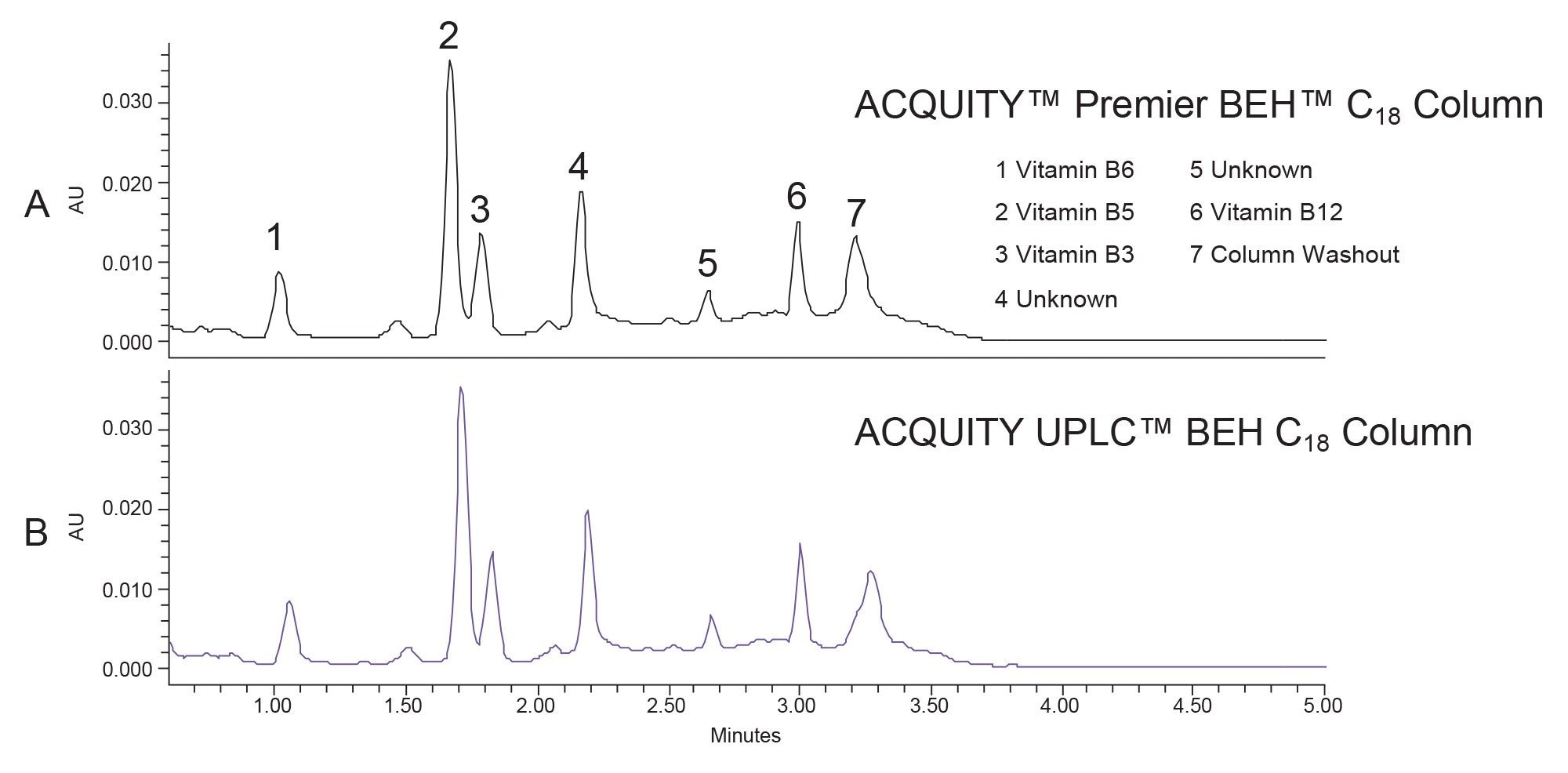
The vitamin beverage was purchased from a local store. All experiments were conducted by injecting aliquots of the neat beverage. Figure 1 shows the B vitamins identified in the sample.

LC Conditions
|
LC systems: |
Waters AutoPurification™ System ACQUITY UPLC H-Class System |
|
UV detection: |
AutoPurification System: 2998 Photodiode Array Detector H-Class System: TUV Detector Wavelength: 270 nm |
|
Columns: |
ACQUITY™ Premier BEH™ C18, 1.7 µm Column, 2.1 x 50 mm, p/n: 186009452 ACQUITY UPLC™ BEH C18, 1.7 µm Column, 2.1 x 50 mm, p/n: 186002350 XBridge™ BEH C18 OBD™ Prep Column, 130 Å, 5.0 µm, 10 x 150 mm, p/n: 186008166 XBridge BEH C18 Premier OBD Prep Column, 130 Å, 5.0 µm, 10 x 150 mm p/n: 186011307 XBridge BEH C18 Premier OBD Prep Column, 130 Å, 3.5 µm, 10 x 100 mm p/n: 186011311 |
|
Column temperature: |
Ambient |
|
Sample temperature: |
Ambient |
|
Sample loop: |
500 µL; stainless steel |
|
Injection volumes: |
As noted in figures |
|
Flow rates: |
Analytical 0.35 mL/min; Prep 5.4 mL/min |
|
Mobile phase A: |
20 mM Ammonium Formate, pH 5.05 |
|
Mobile phase B: |
Methanol |
Gradient Table. Analytical Method
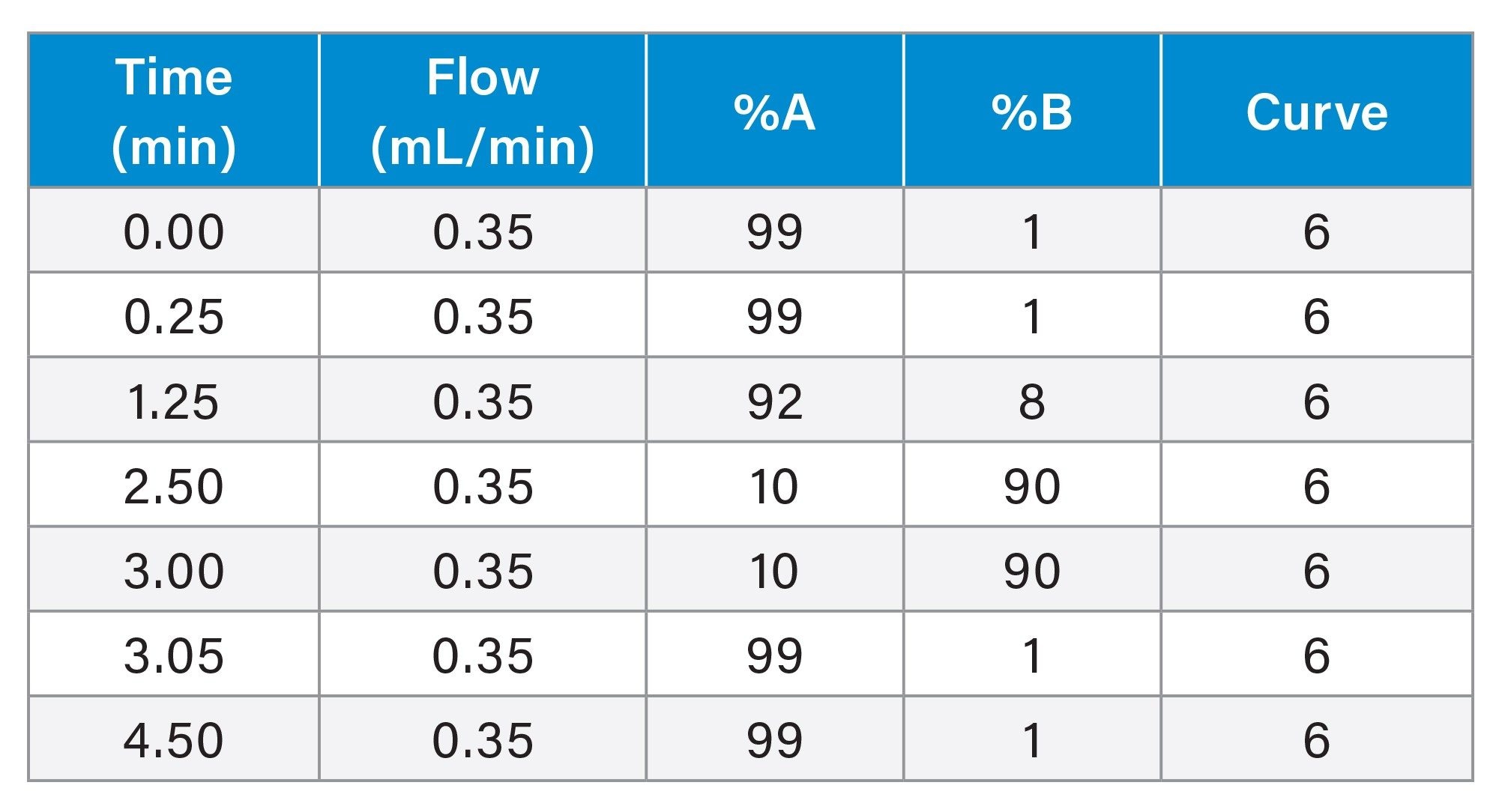
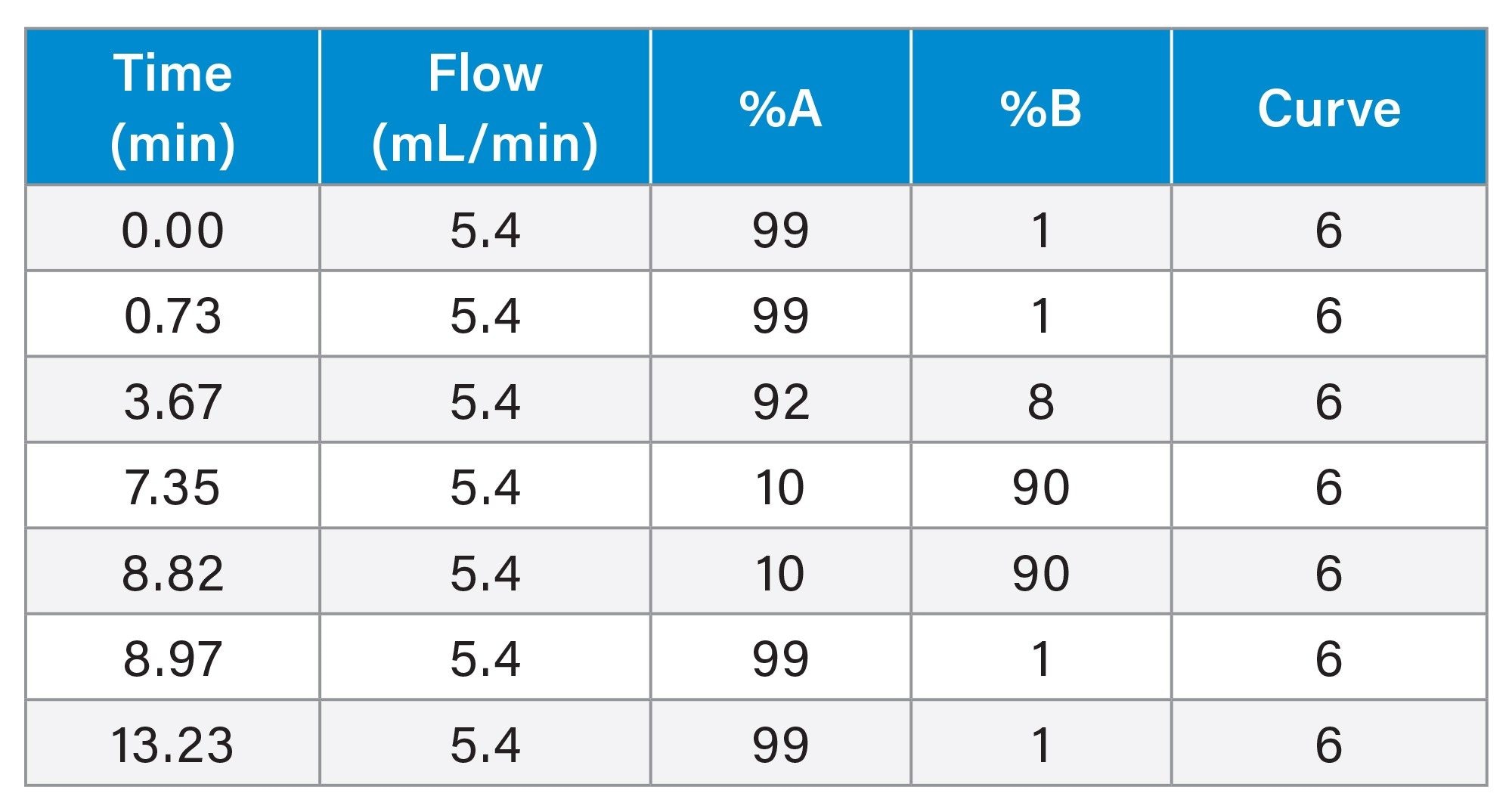
Gradient Table. Preparative Method for 3.5 µm Column
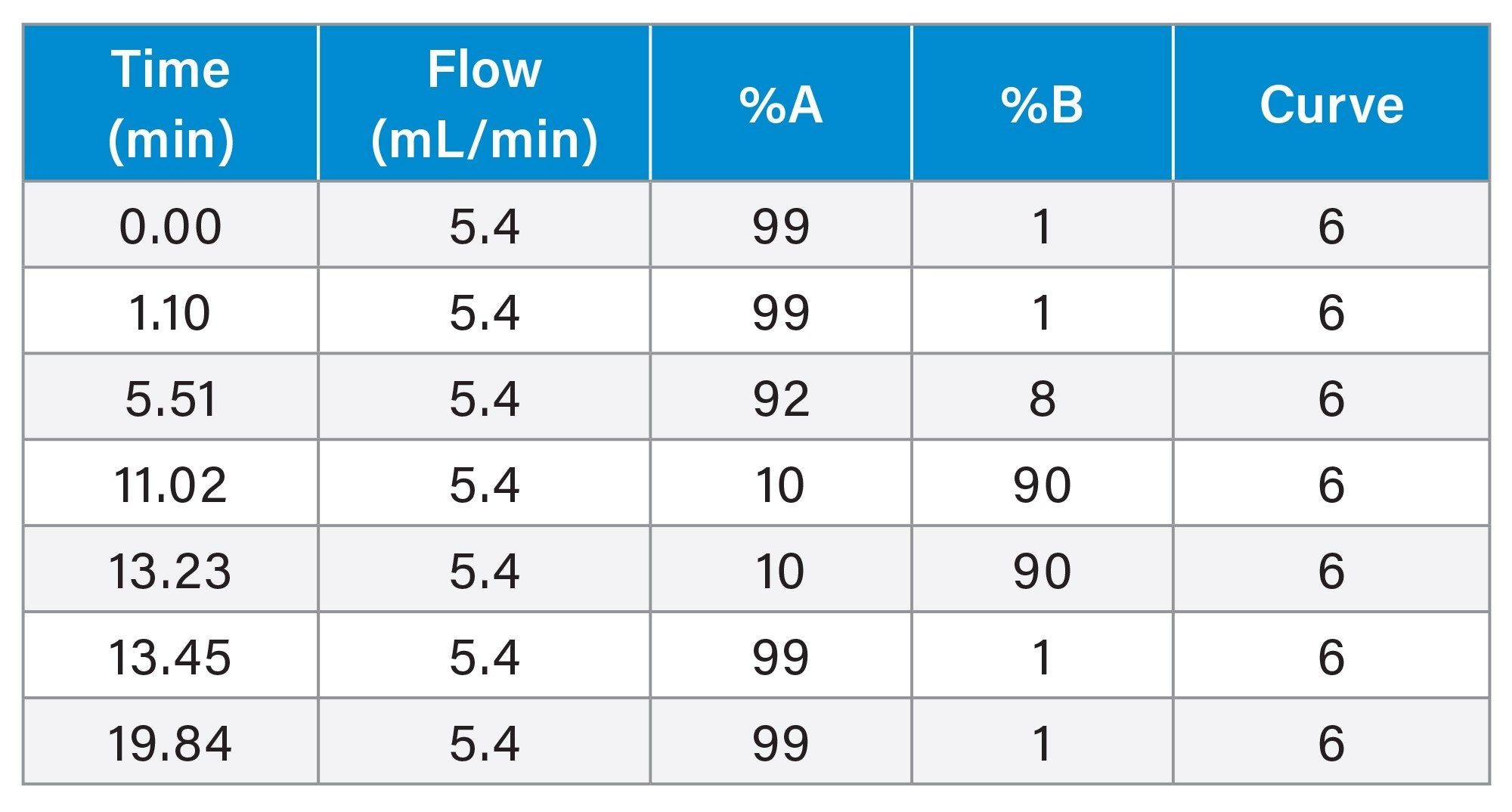
Gradient Table. Preparative Method for 5.0 µm Column
Data Management
|
Chromatography software: |
MassLynx™ version 4.2 |
|
Application manager: |
FractionLynx |
Results and Discussion
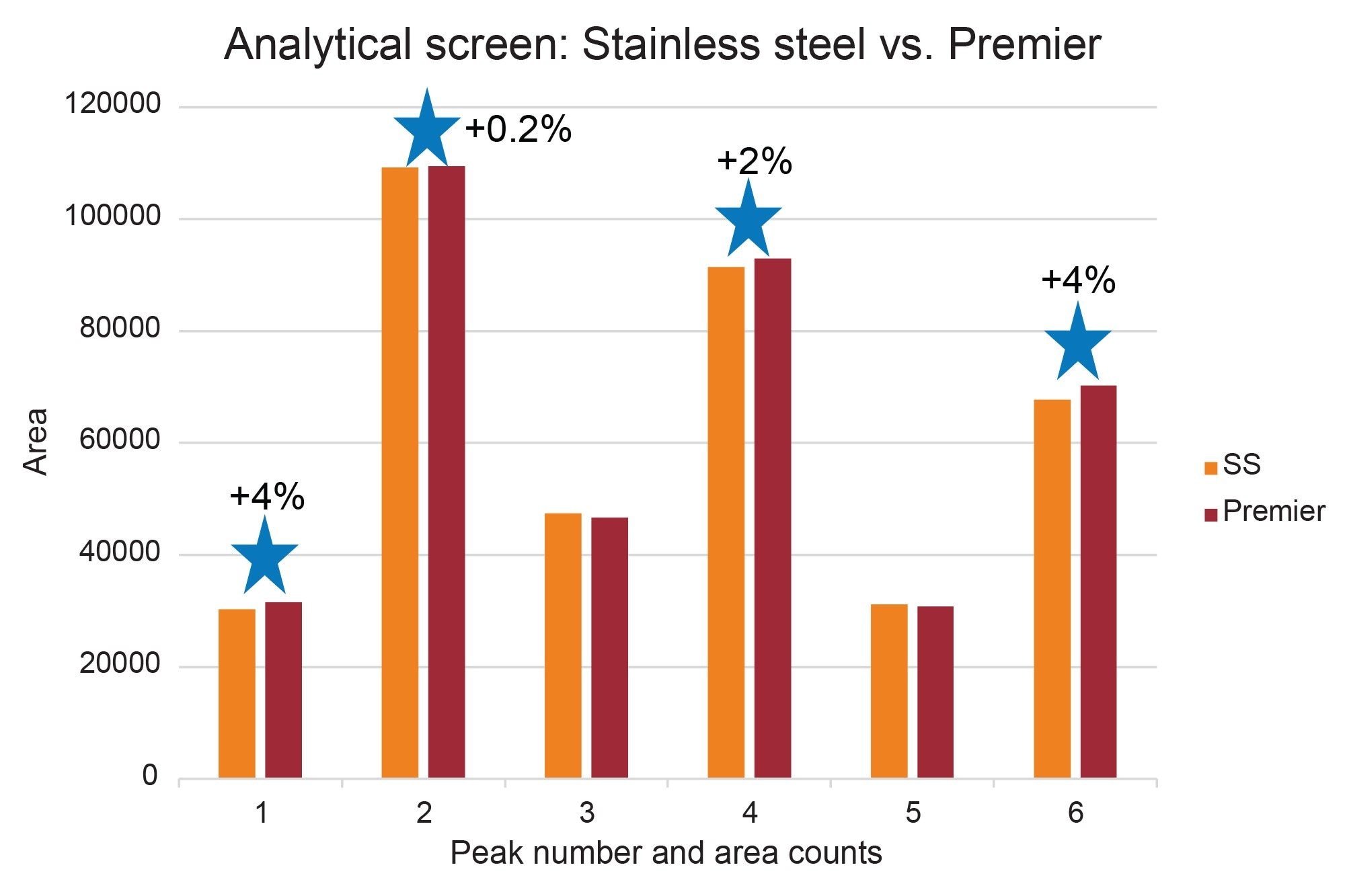
As mentioned above, previous work discussed the enhancement of LC-MS/MS analysis of B-group vitamins using MaxPeak high performance surfaces technology. The reported methodology was modified to illustrate how MaxPeak Premier OBD Preparative Columns could be used for preparative workflows. The vitamin beverage was first analyzed by UPLC using both a stainless steel ACQUITY UPLC BEH C18 Column and an ACQUITY Premier BEH C18 Column. As expected, the chromatographic profile was similar between the two columns (Figure 2). Peak area analysis, as shown in Figure 3, indicated that four of the peaks had higher area counts on the Premier column than on the stainless-steel column. Although the increase in area percent may seem small, the potential for higher yield from the purification may be significant, especially with the trend toward smaller scale isolations in many purification labs today.
The separation was scaled for purification from the 1.7 µm, 2.1 x 50 mm analytical columns to three 10 mm ID XBridge BEH OBD Preparative C18 columns -- a stainless steel, 5 µm, 10 x 150 mm (L/dp 30,000); a Premier, 5 µm, 10 x 150 mm; and a Premier, 10 x 100 mm, 3.5 µm (L/dp 28,571). Scaling between columns with different particle sizes requires the L/dp ratio (the ratio of the column length to the particle diameter) be similar to maintain the separation.7 The system volume on the H-Class System was determined to be 0.378 mL, while the system volume on the AutoPurification System was measured at 1.71 mL. The analytical method was scaled for prep, as shown in Tables 1, 2, and 3. The flow rate was standardized at 5.4 mL/min for both the 3.5 and 5 µm columns for two reasons. Scaling the flow rate of 0.35 mL/min on the 1.7 µm, 2.1 x 50 mm column to the 5 µm, 10 x 150 mm column would require a flow rate of only about 2.7 mL/min, giving an exceedingly long run time of almost 40 minutes. Increasing the flow rate to 5.4 mL/min, reduced the run time to just under 20 minutes, a much more reasonable preparative run time. Although the scaled flow rate for the 3.5 µm, 10 x 100 mm column would be 3.8 mL/min, increasing the flow rate to 5.4 mL/min saved over 5 minutes per run. Therefore, to save preparative run time, the custom flow rate of 5.4 mL/min was used for both the 3.5 and 5 µm 10 mm ID columns.
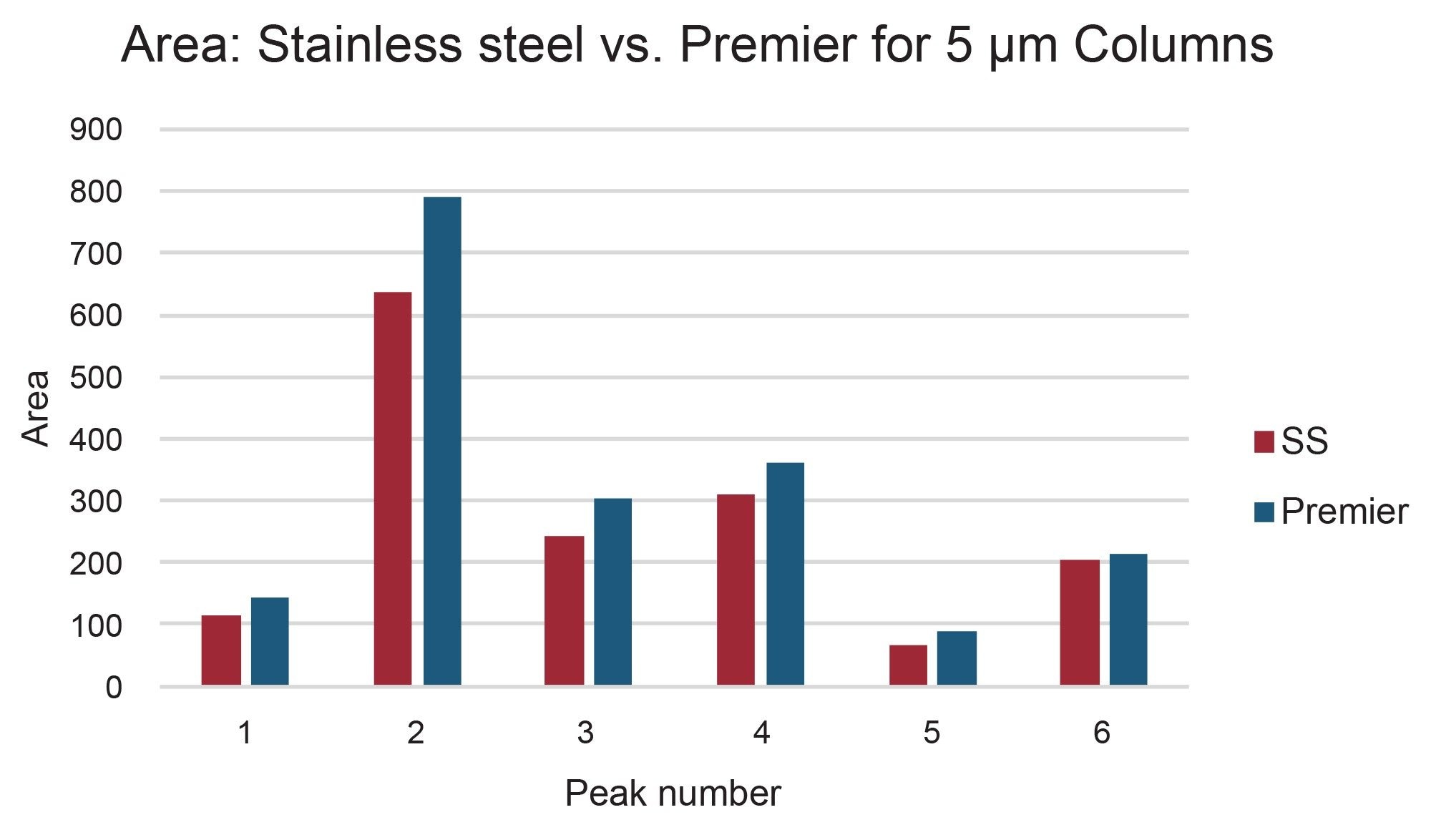
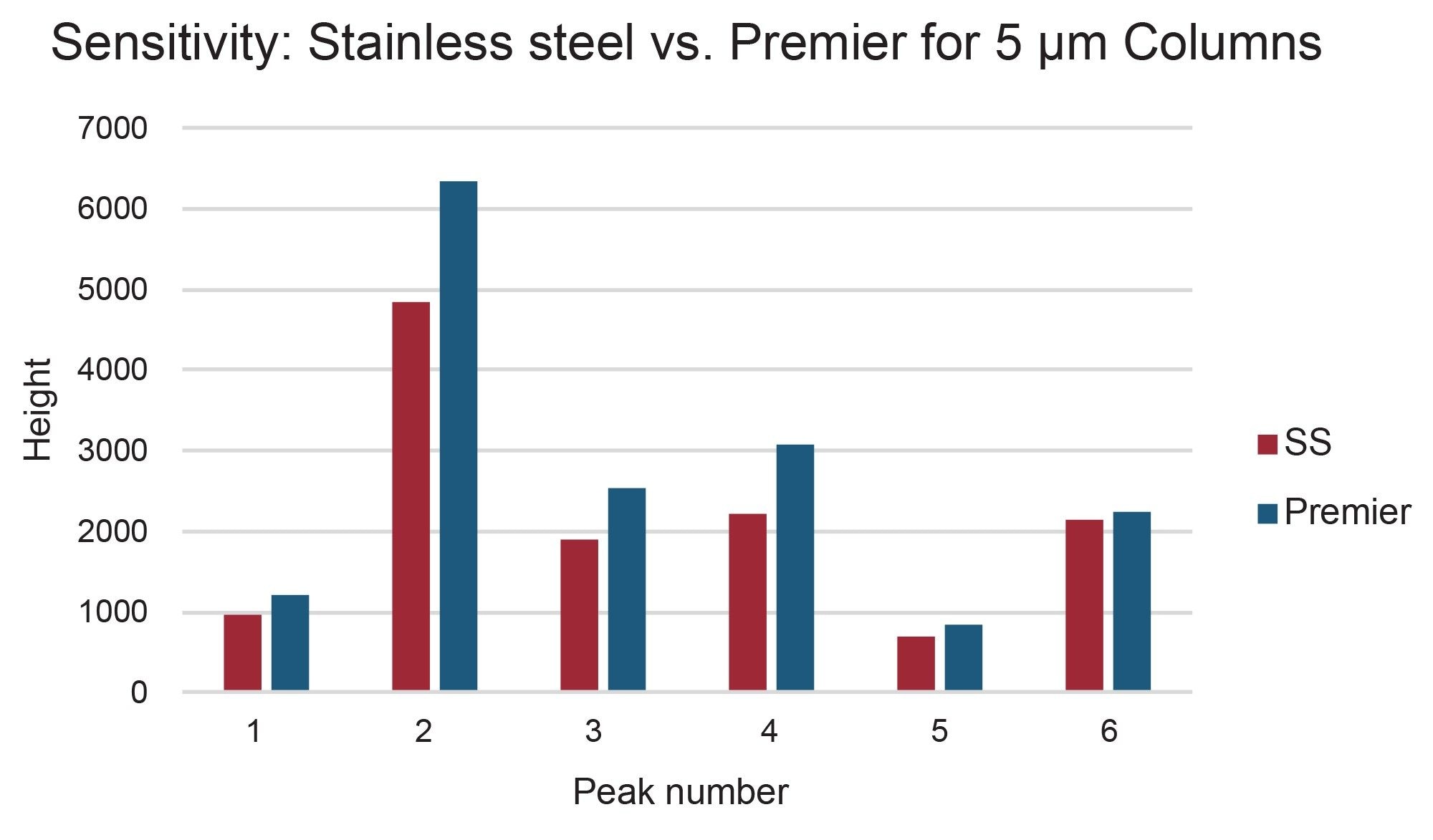
The preparative chromatograms from isolations performed on the 5 µm, 10 x 150 mm XBridge BEH C18 stainless steel and XBridge Premier BEH C18 OBD Columns are shown in Figure 4. The chromatographic profiles were quite similar for these early injections on these new columns, but upon closer inspection, the Premier column provided increased areas (~5–25%) as compared to the stainless-steel column for all 6 peaks of interest. Likewise, the Premier column showed higher sensitivities (~5–30%) as compared to the stainless-steel column for all the peaks, as shown in Figures 5 and 6. The stainless-steel column was conditioned after a few injections, and the peak response differences between it and the Premier versions was less after several injections. It is important to note that in those laboratories where low/high pH method switching is common practice for the analysis and purification of large libraries, the high pH methods may strip the conditioning from the stainless-steel column. With the first subsequent low pH injections occurring after the high pH methods, the stainless-steel column areas and/or sensitivities are most likely to be lower than those for the Premier column because the metal oxide sites in the column have been re-exposed. So, in effect, high pH purifications will most likely “clean” the column of non-specific adsorbers, leading to the possibility that the next sample injection may show reduced sensitivity or poor peak shape because of the presence of non-specific adsorbing compounds. Premier columns with high-performance surface technology (aka HPS™ Technology) need no conditioning and perform consistently from the first injection. For scientists who only have enough crude sample for one preparative injection, the need to have good peak shape, high peak areas, and sensitivities is crucially important and can mean the difference between purification success and sample loss.
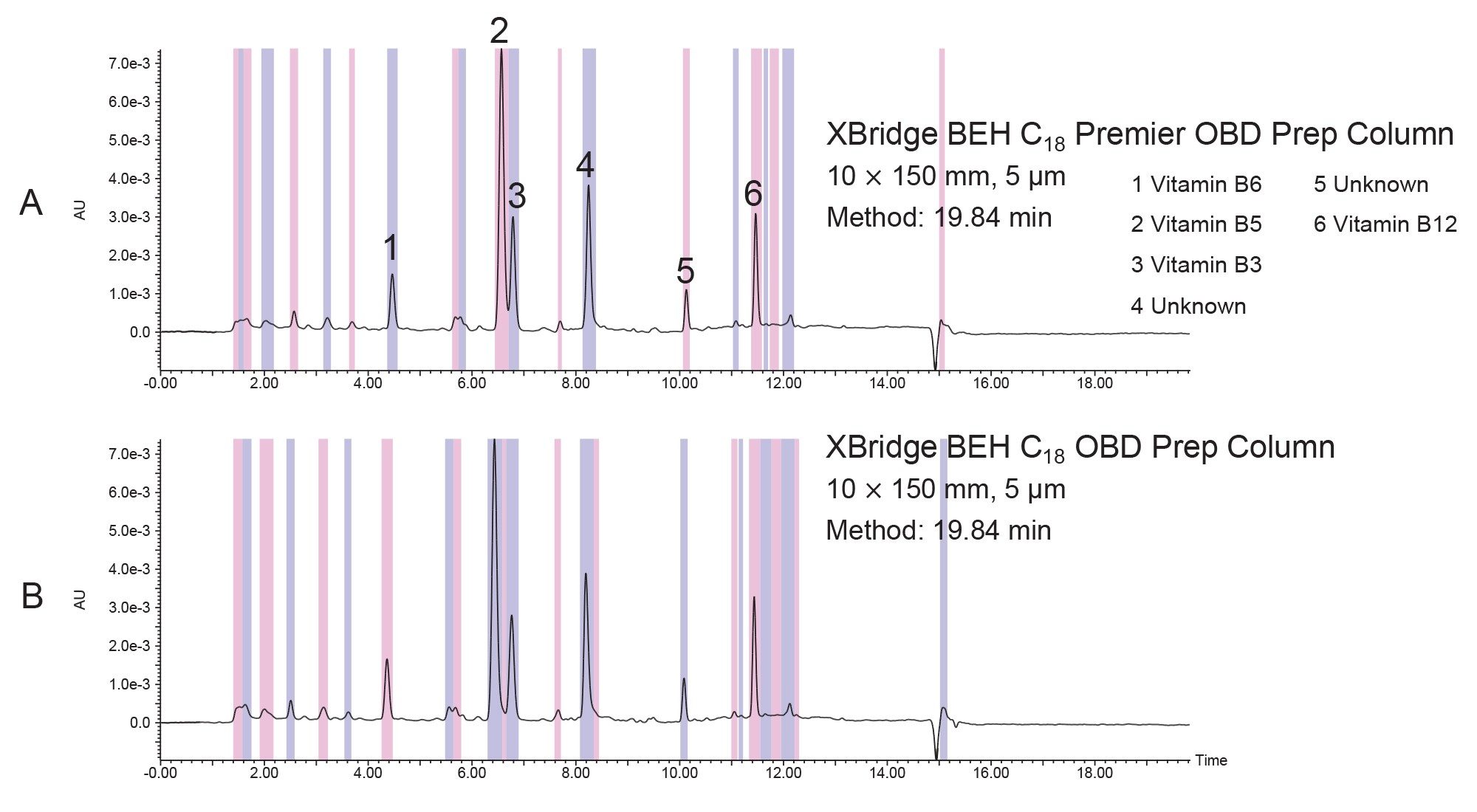
The interest in small particle preparative separations is becoming more prevalent as pharmaceutical companies synthesize an ever-increasing number of new chemical entities for drug development. As the need grows to isolate highly pure compounds faster at increasingly reduced scale without sample loss, preparative chromatography with HPLC columns packed with smaller stationary phase particles can be critical to employ. Figure 7 shows the preparative chromatography comparison between the 5 µm 10 x 150 mm Premier OBD column (L/dp 30,000) and the 3.5 µm 10 x 100 mm Premier OBD column (L/dp 28,571). Clearly, the separations are analogous since care was taken to ensure similar L/dp ratios during scaleup, but the 3.5 µm column has narrower peaks (Figure 8) and a run time which is reduced by 6.6 minutes. Reduced run times ultimately result in lower solvent consumption, less waste, and decreased time required for downstream sample handling. These savings translate to reduced overall process cost.
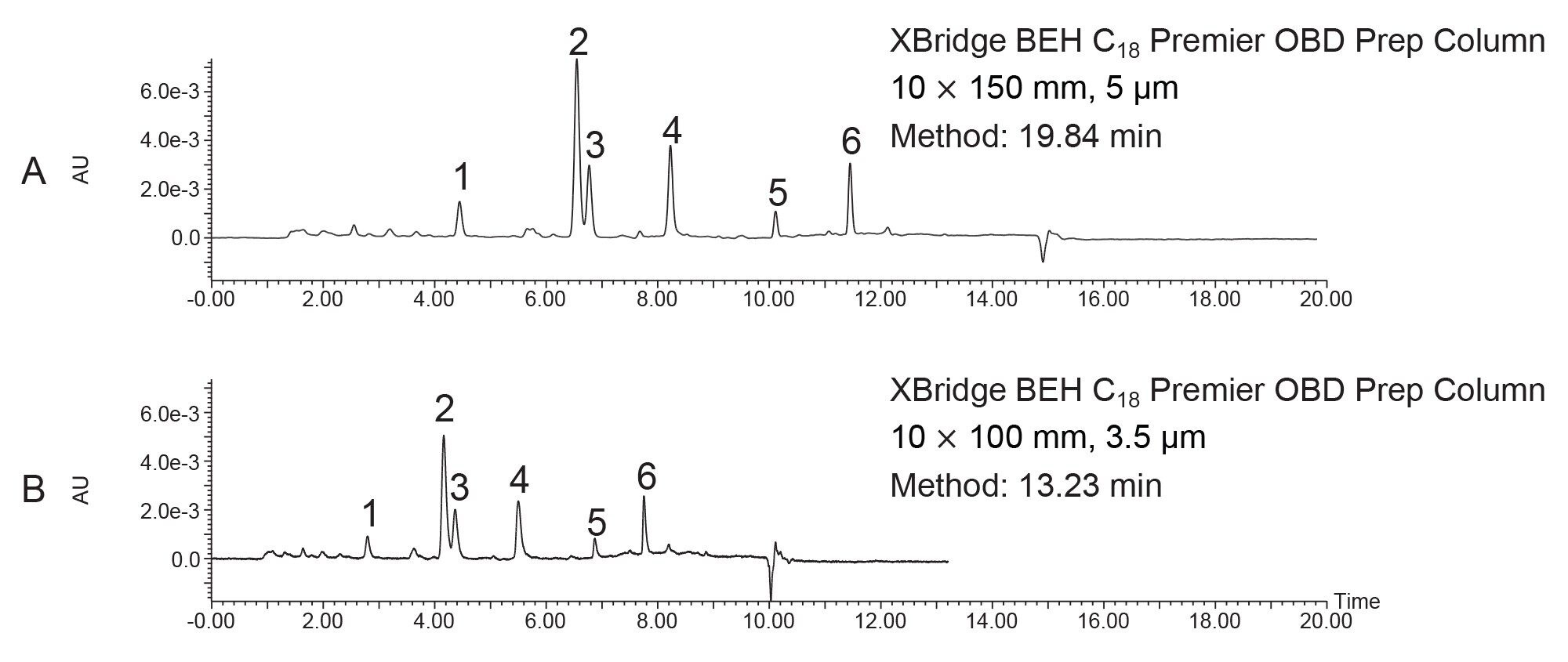
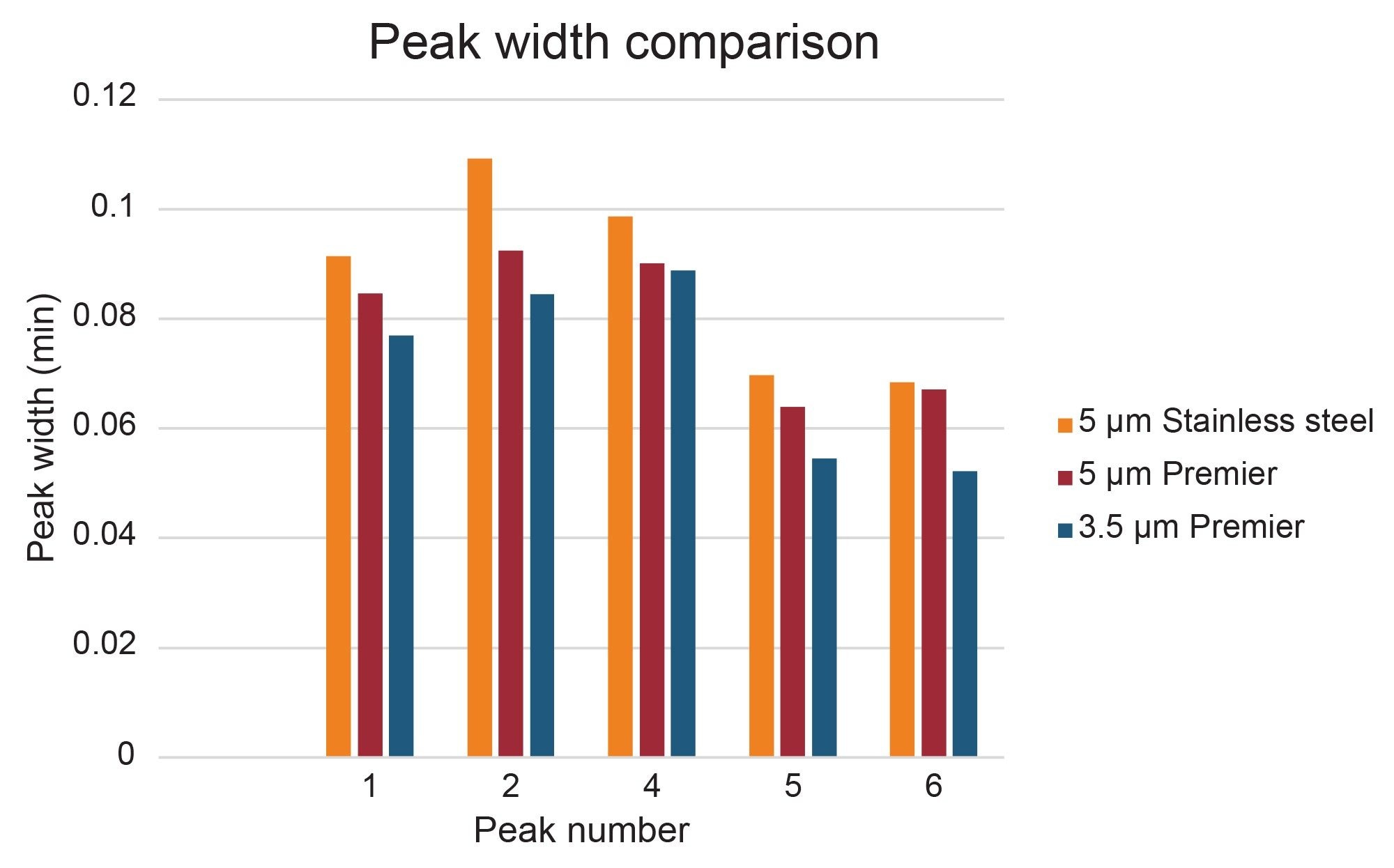
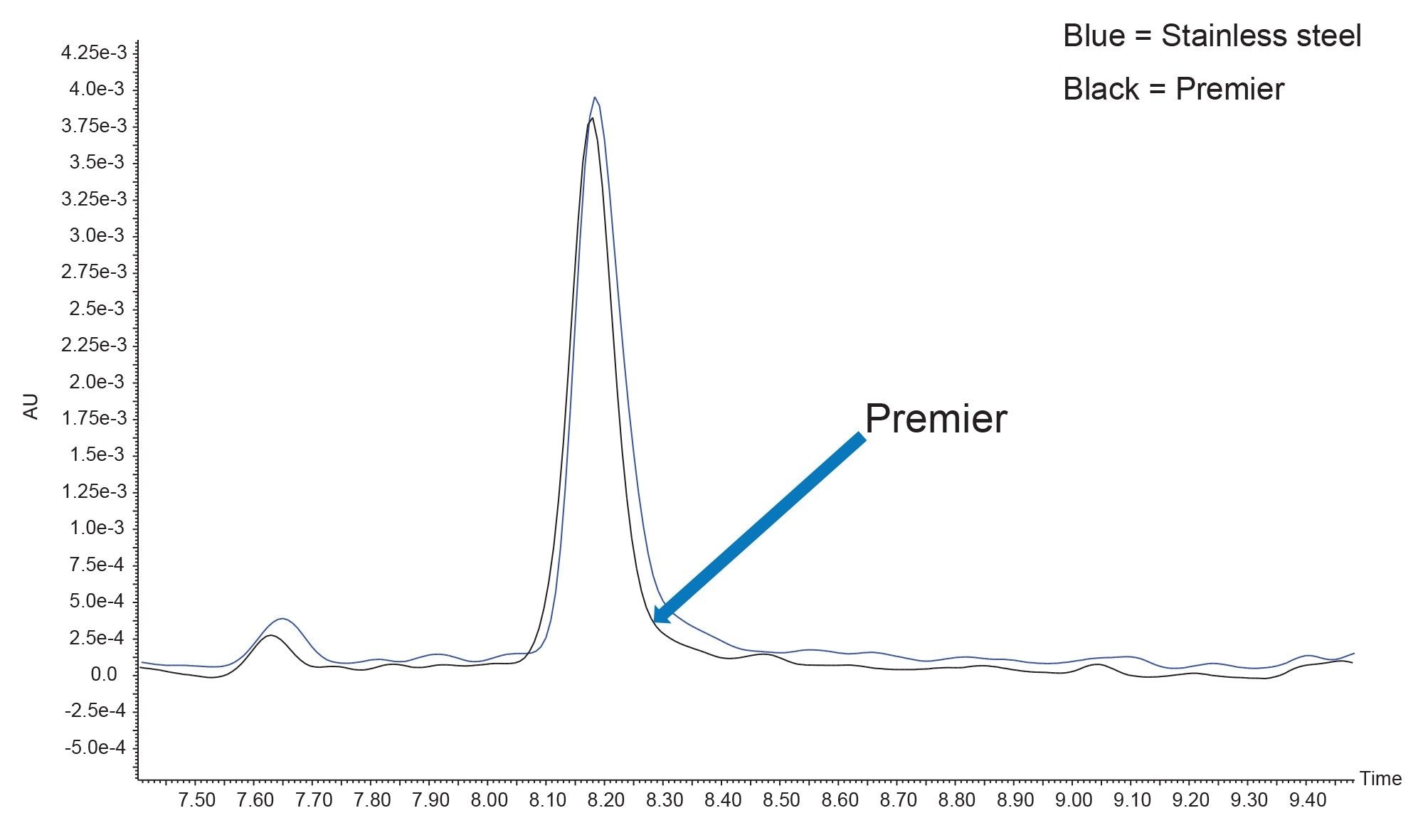
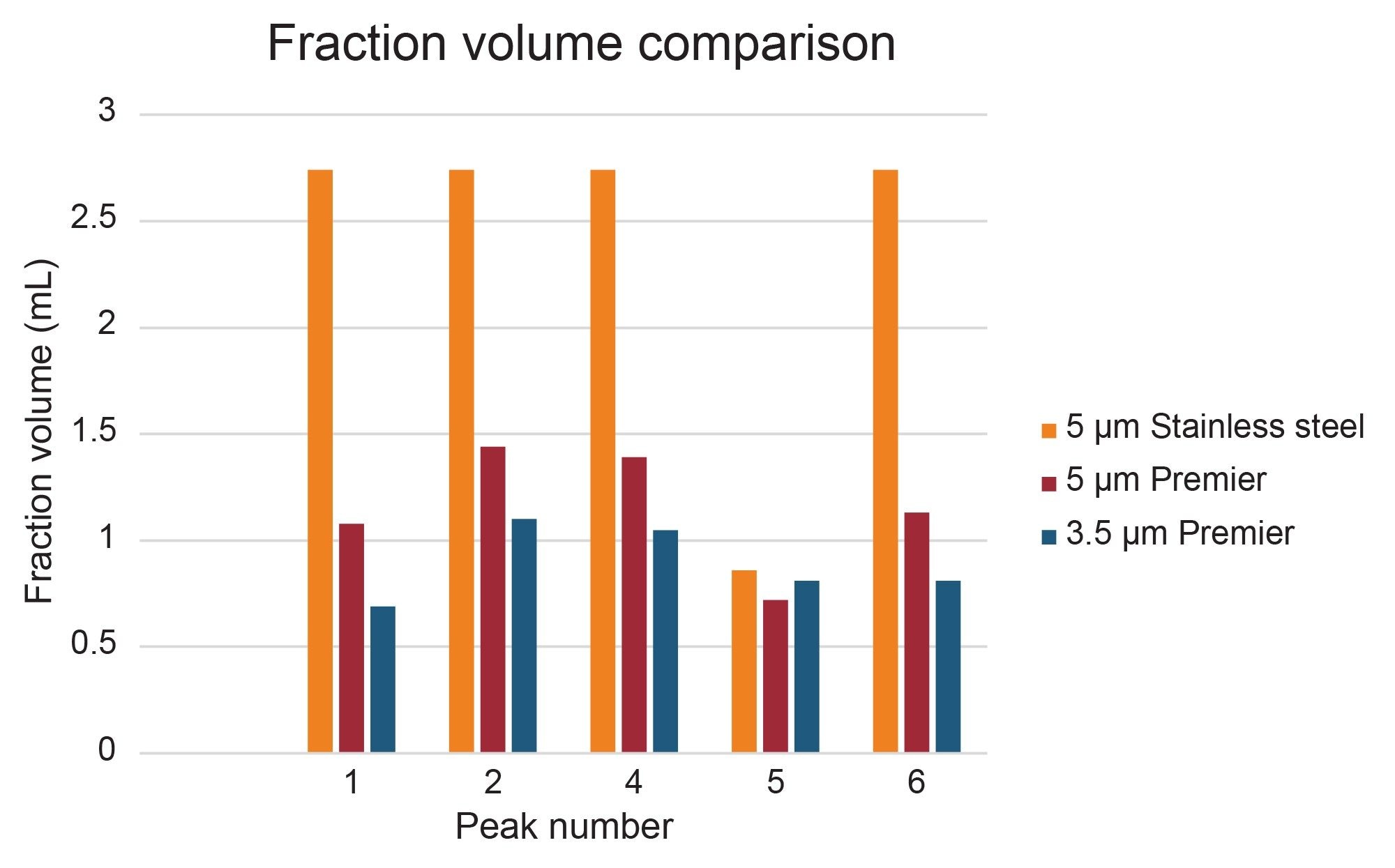
Peak shape and width impact fraction collection in preparative isolations. Narrow peaks reduce fraction volumes and lead to more efficient downstream processing by decreasing the amount of time needed to dry down fractions to recover the desired product. Different compound properties and column attributes influence peak shape, but interestingly, a comparison of peaks 1, 2, 4, 5, and 6 in this separation shows that they were narrower (peak width measured at half-height) on the 5 µm Premier OBD preparative column than on the 5 µm stainless steel column (Figure 8). Figure 9 illustrates one representative chromatographic example of the difference in peak width (for Peak 4) in the preparative separations performed on the 5 µm, 10 x 150 mm stainless steel and Premier OBD preparative columns. Narrower peaks subsequently translate to lower fraction volume from collection. The fraction volume for Peak 4 on the stainless-steel column was 2.74 mL, while the fraction volume was 1.39 mL on the Premier column. This ~50% reduction in fraction volume would impact dry down time substantially and decrease the amount of time required to advance the product on to the next step in the purification workflow. The differences in fraction volume between the 5 µm, 10 x 150 columns, as well as the 3.5 µm, 10 x 100 mm column are presented in Figure 10.
Conclusion
The high-performance surface technology inherent in MaxPeak Premier columns (Waters HPS™ Technology) reduces the unwanted interactions between metal-sensitive compounds with strong acidic groups like sulfates, phosphates, or carboxylic acids from adsorbing to the metal surfaces within the column. Non-specific adsorbing compounds may exhibit poor peak shape or reduced sensitivity and create concern when isolating target molecules, especially those present in low quantities. In these experiments, we demonstrated the advantages that MaxPeak Premier OBD Preparative Columns provide in the purification workflow, using a vitamin beverage for the isolation of targets (in this case, four B vitamins and two unknowns) in a complex matrix.
As shown in these experiments, for certain compounds, 5 µm MaxPeak Premier OBD Preparative Columns can decrease peak widths as compared to 5 µm stainless-steel prep columns. 3.5 µm MaxPeak Premier OBD Preparative Columns can also be used to improve purification workflow efficiency by reducing chromatographic run times and fraction collection dry-down times due to the narrower peaks obtained with the smaller particles. Enhanced sensitivity for greater confidence in detecting and isolating impurities at low levels in crude sample mixtures make MaxPeak Premier OBD Preparative Columns especially valuable for the purification of compound libraries where first-time injection success is paramount. In high-throughput laboratories, large numbers of samples must be purified efficiently (often in only one injection), while saving both precious sample as well as process time. MaxPeak Premier OBD Preparative Columns are well-suited for assisting the purification scientist to meet such demands.
References
- Yang J, Rainville P. Enhancing the LC-MS/MS Analysis of B-group Vitamins with MaxPeak High Performance Surfaces Technology. Waters Corporation; Waters Application Note. 720007264. 2021.
- Waters Corporation, A Review of Waters’ New Hybrid Particle Technology and Its Use in High Performance Liquid Chromatography (HPLC), White Paper. WD164. 1999.
- Waters Corporation, A Review of Waters’ Hybrid Particle Technology, Part 2 Ethylene-Bridged [BEH Technology™] Hybrids and Their Use in Liquid Chromatography, White Paper. 720001159. 2004.
- Waters Corporation, Topics in Liquid Chromatography, Part 2: Optimum Bed Density [OBD™] Columns: Enabling Technology for Laboratory-Scale Isolation and Purification, White Paper. 720001939. 2012.
- DeLano M, Walter T, Lauber M, Gilar M, Jung M, Nguyen J, Boissel C, Patel A, Bates-Harrison A, Wyndham K, Analytical Chemistry, https://doi.org/10.1021/acs.analchem.0c05203
- Iqubal A, et.al., RSC Adv., 6 (2016) 68574.
- Jablonski J. 5 Rules of Scaling LC Purification. Waters Corporation; Article. 720008383. 2024.
720008691, January 2025