SPE is Chromatography
Keep in mind that solid-phase extraction has the same fundamental basis as HPLC. Any knowledge of the chromatographic behavior of the analytes of interest, and of other matrix components, can help in choosing the proper sorbent and eluents. If, for example, you know that certain chromatographic conditions provide excellent separation of your analyte from interferences, then you may choose a similar SPE sorbent and solvent combination. Similarly, if you are trying to remove an interference that coelutes in HPLC, then you know a priori that similar SPE conditions will not be successful.
General Elution Protocols
There are two general strategies for isolating and cleaning up sample components of interest:
The first strategy is usually chosen when the desired sample component is present in high concentration. When components of interest are present at low levels, or multiple components of widely differing polarities need to be isolated, then the second strategy is generally employed. Trace enrichment of compounds present at extremely low levels and concentration of dilute samples are also achieved by the second strategy.
Steps of a Solid-Phase Extraction Procedure
The following section describes the steps involved in a complete solid-phase extraction procedure. In many applications, one or more of the steps, listed below and subsequently described by general examples, can be omitted, thereby simplifying the procedure. The procedures illustrated here use samples containing dyes so that separations may be easily visualized. Keep in mind that most samples contain colorless components that require some type of detector or test to locate them in the collected fractions. Use the following information as a guideline in the development of your own procedure or when modifying procedures published in the literature.
Principal Separation Modes in Solid-Phase Extraction [SPE]
Normal-Phase Chromatography
This mode is classically used to separate neutral organic compounds whose chemical nature ranges from hydrophobic to moderately polar.
To perform normal-phase chromatography with SPE cartridges, use a step gradient of nonpolar solvents with a polar packing material.
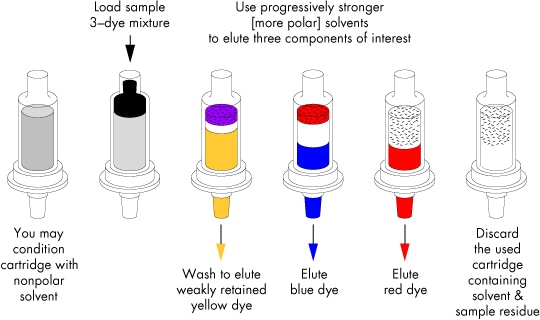
This procedure is illustrated in the figure below for a sample containing a mixture of three neutral, relatively non-polar organic dyes [yellow, red, and blue] that appears black when initially loaded onto the cartridge bed.
Illustration of a General Elution Protocol for Normal-Phase Chromatography on SPE Cartridges
(Silica, Florisil, Alumina, Diol, CN, NH2)
Reversed-Phase Chromatography
Because of the multiplicity of aqueous samples spanning a breadth of applications from environmental water to fruits and vegetables, from beverages to biological fluids, reversed-phase chromatography has become the predominant mode of SPE.
To perform reversed-phase chromatography with SPE cartridges, use a gradient of strongly to weakly polar solvents [from weak to strong solvent elution strength] with a non-polar packing material.
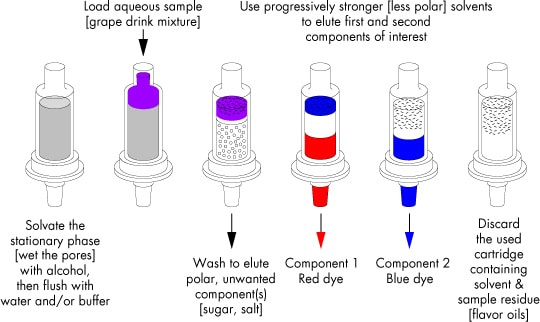
This procedure is illustrated in the figure below for a sample of an aqueous grape drink containing two polar food dyes [red and blue], as well as sugar and artificial flavor [but no real grape juice!]. As prepared, this drink appears light purple in a glass, since the dye concentration is dilute. When a portion is loaded onto a prepared SPE cartridge, the strongly retained dyes become concentrated near the inlet in a dark purple band.
Illustration of a General Elution Protocol for Reversed-Phase Chromatography on SPE Cartridges
(C18, tC18, C8, CN, Diol, HLB, Porapak RDX, NH2)
Ion-Exchange Chromatography
Compounds that are ionic or ionizable are often best isolated using some form of ion-exchange chromatography. This separation mode is orthogonal to the more widely used normal-phase and reversed-phase modes and provides a powerful, selective second dimension to sample preparation protocols.
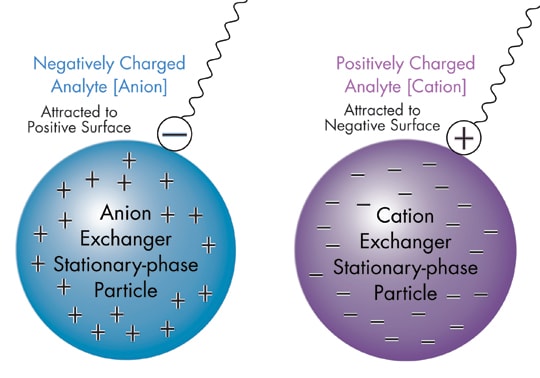
Illustration of the Two Major Types of Phases—Anion and Cation Exchange—
and How They Selectively Attract and Retain Molecules of Opposite Charge
To perform ion-exchange chromatography with SPE cartridges, use a gradient of pH or ionic strength with an ion exchange packing material.
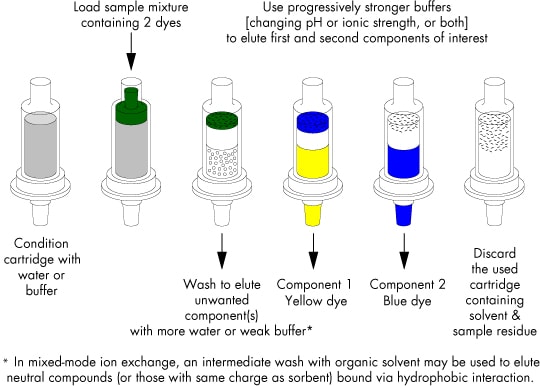
This procedure is illustrated in the figure below for a sample of an aqueous mixture of two ionic dyes with different pKa values. When loaded onto the cartridge, both are strongly retained, and the combination of blue and yellow components appears as a green band near the inlet.
Illustration of General Elution Protocol for Ion-Exchange Chromatography on SPE Cartridges
(NH2, Accell™ Plus QMA, Accell Plus CM, SCX, SAX, WCX, WAX)
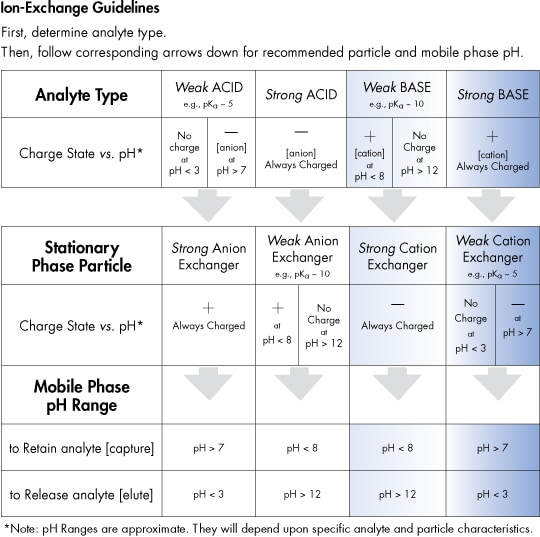
Cation and anion exchangers are further categorized as either weak or strong exchangers, depending upon the type of ionic group on their surface. Strong cation exchangers possess an acidic surface moiety such as a sulfonic acid that is always ionized [negatively charged] over the whole pH range. Weak cation exchangers possess an acidic surface moiety such as a carboxylic acid that is negatively charged at high pH but neutral at low pH. Similarly, strong anion exchangers typically bear quaternary ammonium groups that are always positively charged, while weak anion exchangers possess primary, secondary, or tertiary amine groups that may be positively charged at low pH but neutral at high pH.
Use the following table as a guideline to choose the appropriate SPE ion-exchange cartridge type for your particular analyte.
Mixed-mode ion exchange chromatography combines the use of reversed-phase and ion-exchange modes into a single protocol on a single SPE cartridge. It can be used to isolate and separate neutral, acidic, and basic compounds from a single complex matrix. An ideal mixed-mode SPE sorbent substrate remains water-wettable while exhibiting strong reversed-phase retention of hydrophobic compounds. On its surface are ion-exchange functionalities of one of the four general types just described above. Intermediate washes with organic solvent mixtures of appropriate elution strength may be used to isolate neutral compounds [including ionizable analytes in their neutral state]. Selective elution of ionically bound analytes may be attained by manipulating the charge of either the analyte [when bound to strong ion exchangers] or of the sorbent [for analytes bound to weak ion exchangers].
| < Previous | Next > |