Improvements in the Analysis of Synthetic Peptide Enfuvirtide and HAART Drugs Utilizing MaxPeak™ High Performance Surfaces (HPS)
Abstract
HIV is a virus that affects millions of people every year. If left untreated, it can progress into AIDS, which causes those infected to be further immunocompromised and susceptible to various ailments and cancer. AIDS is the cause of death for hundreds of thousands of people every year. There have, however, been advancements in treatment options for HIV. These treatments include HAART combined with synthetic peptides such as enfuvirtide.
To address the need for a rapid, modern analytical method capable of separating the current standard of HIV treatment, we developed a linear and reproducible HPLC-UV/MS method that facilitates separations in under 6 minutes. Further, we demonstrated the benefits of using MaxPeak HPS, when compared to traditional stainless-steel systems, for synthetic peptide analysis.
Benefits
- MaxPeak HPS is shown to be more reproducible compared to traditional stainless-steel systems
- Max Peak HPS offers increases in area and height signals for enfuvirtide, leading to lower limits of quantitation
- A single method has been developed to quantify both HAARTs and Enfuvirtide with a run time nearly four times faster than previous methods
Introduction
Human immunodeficiency virus (HIV) affects tens of millions of individuals, young and old, worldwide.1 If left untreated, HIV can progress into acquired immunodeficiency syndrome (AIDS), a disease that destroys the immune system of those infected, leaving them vulnerable to a wide variety of other diseases and cancer.2,3 While there is no cure for HIV or AIDS, HIV treatment has come a long way. Currently, HIV patients have access to a variety of highly active antiretroviral therapy (HAART) drugs, which typically combine different antiretroviral drugs to get the best therapeutic effect for each individual patient.4,5 If the patient is unresponsive to HAART, sometimes another drug is prescribed to improve outcomes. Enfuvirtide is one of the candidates that can be used in combination with HAART drugs.6 Enfuvirtide is not a small molecule like the antivirals, but rather a synthetic peptide.7
Since enfuvirtide is always used in combination, the quantitation and separation of enfuvirtide in a solution containing HAART drugs in the current standard of care is important. In this application note, we describe a linear and reproducible method that covers the current panel of HAART including enfuvirtide using HPLC-UV/MS. The concept of a multidrug analysis method is not novel, however, of the current methods available for separating and quantitating HAARTs, none to our knowledge have been updated to include enfuvirtide.8,9 In addition, other methods have been shown to take nearly 20 minutes to fully separate a panel of HAARTs, whereas here we were able to complete the separation in under 6 minutes. Further, Waters™ HPS has been shown to mitigate some undesirable metal and peptide interactions that can potentially lead to lower responses in chromatography.10
Experimental
Stock Standard Preparation
Enfuvirtide acetate was purchased from Sigma Aldrich (Saint Louis, MO). Dolutegravir and bictegravir were purchased from Selleck Chem (Houston, TX). Tenofovir, lamivudine, emtricitabine, and abacavir sulfate were purchased from Cayman Chemical (Ann Harbor, MI). Stock concentrations varied based on solubility and amount of material purchased (Table 1). Any salt factors were taken into consideration during preparation. Sometimes sonication was used to help coax standards into solution.
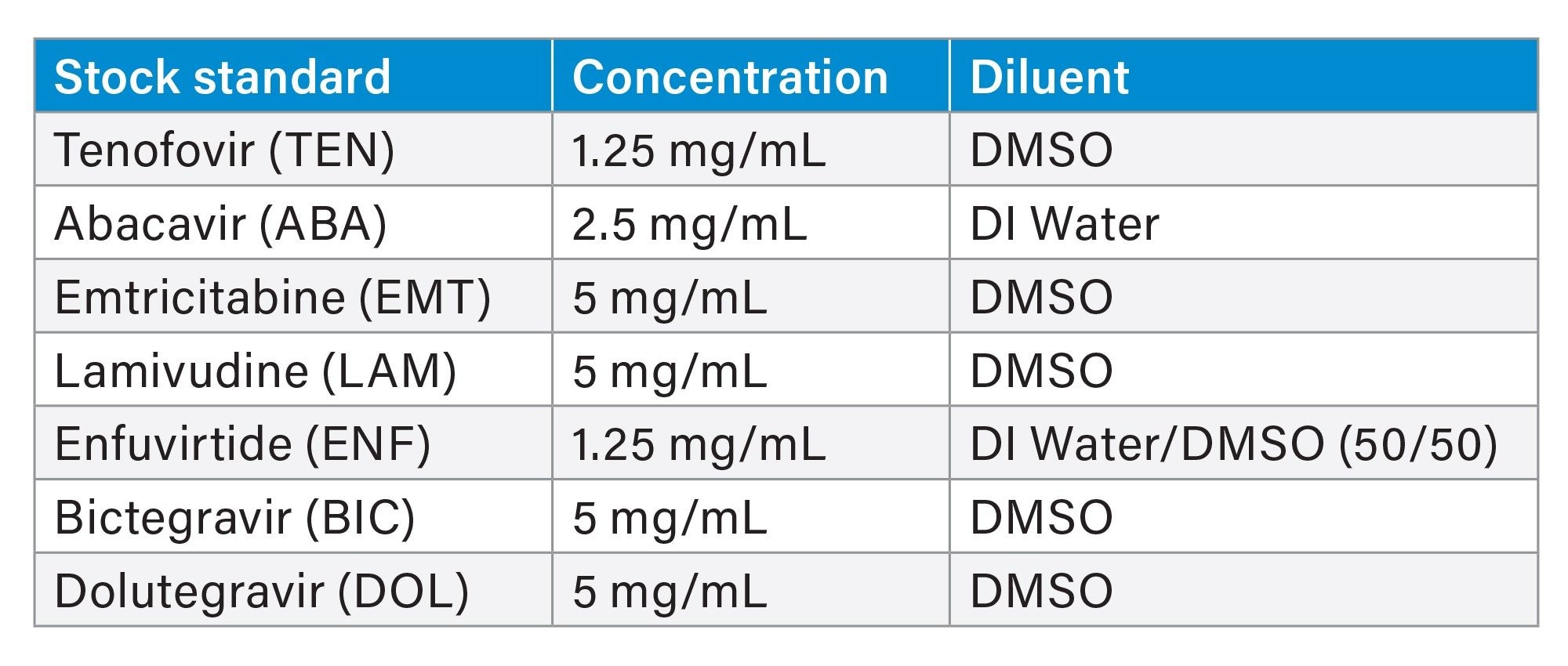
DI Water is Deionized water. DMSO is Dimethyl-Sulfoxide.
Stocks were stored at 2 °C–8 °C. Given the physical properties of DMSO, stocks solidified in cold storage. Thawed stocks were allowed to equilibrate to ambient room temperature prior to standard preparation.
System Suitability Standard Preparation:
Stocks were diluted using 5% acetonitrile (ACN) in DI Water. Stocks were combined and prepared so that all analytes were at a 100 µg/mL concentration (HIV Drug Mix Standard).
Further, a separate standard containing only enfuvirtide was made to examine the method performance exclusively on the synthetic peptide. This standard was created by diluting the enfuvirtide standard to a 100 µg/mL concentration using 5% ACN in DI water (Enfuvirtide Suitability Standard).
Linearity and Limit Standards Preparation:
A combined HIV Drug Mix Standard (excluding enfuvirtide) was prepared at a 250 µg/mL using the 5% ACN diluent. The standard underwent subsequent dilutions using 5% ACN in DI water to make various calibration points for each for the HIV antiretroviral drugs in the mixture.
A separate and exclusive calibration curve was made for enfuvirtide. The enfuvirtide calibration standards were created by first making an enfuvirtide standard at a 1000 µg/mL concentration using 5% ACN in DI Water. The standard underwent subsequent dilutions using 5% ACN in DI water to make various calibration points for enfuvirtide.
This standard was also used to make the limit of quantitation (LOQ) standards used to compare MaxPeak HPS and traditional stainless-steel system systems.
Method Conditions:
Due to the differences in UV absorbances of peptides and antiretroviral pharmaceuticals, two wavelengths were used for analysis.6,11 Wavelength 214 nm was used for all quantitative purposes for enfuvirtide, while 260 nm was used for quantitative data for the antiretroviral pharmaceuticals.
LC Conditions
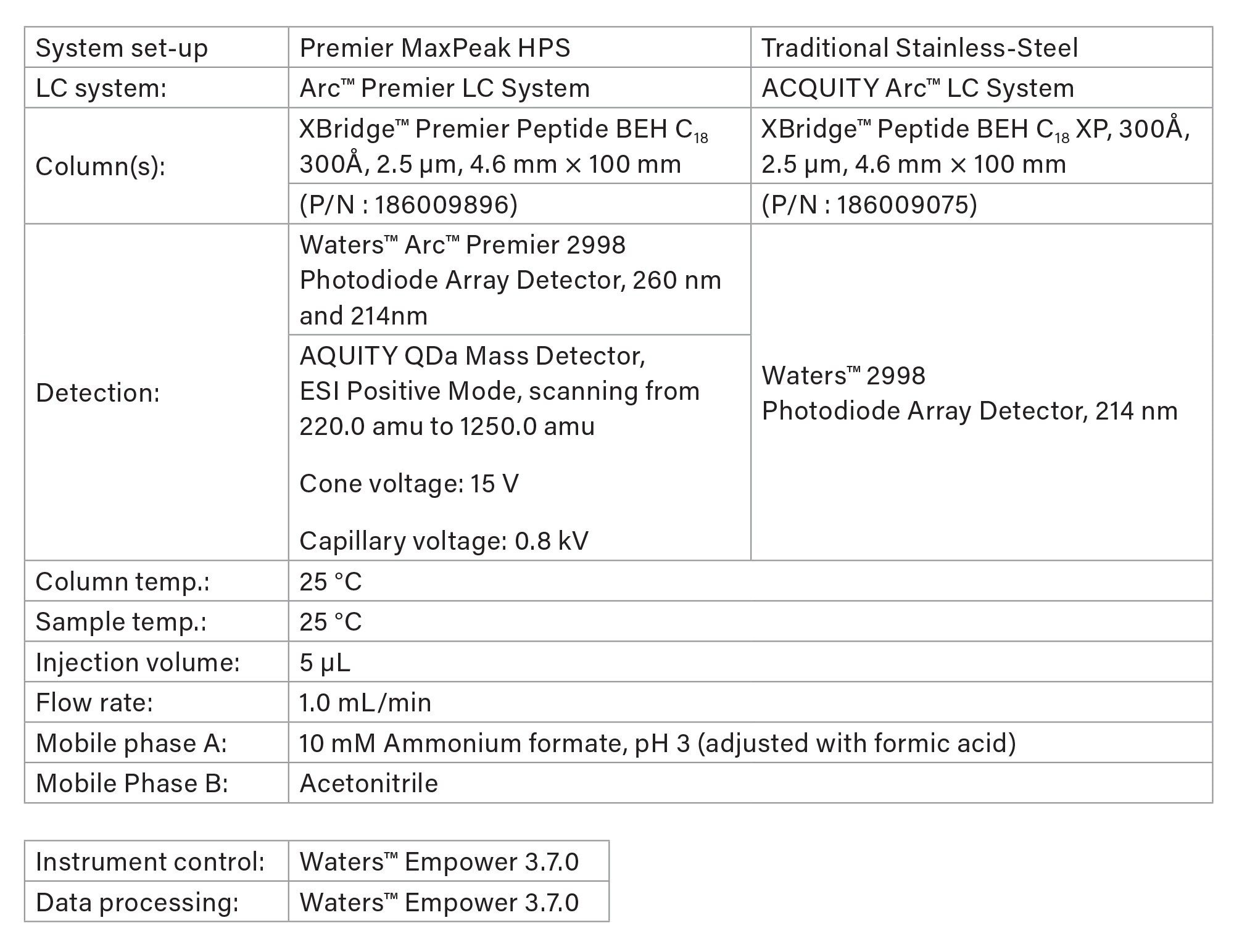
Gradient Table
Results and Discussion
Method of Separation Results:
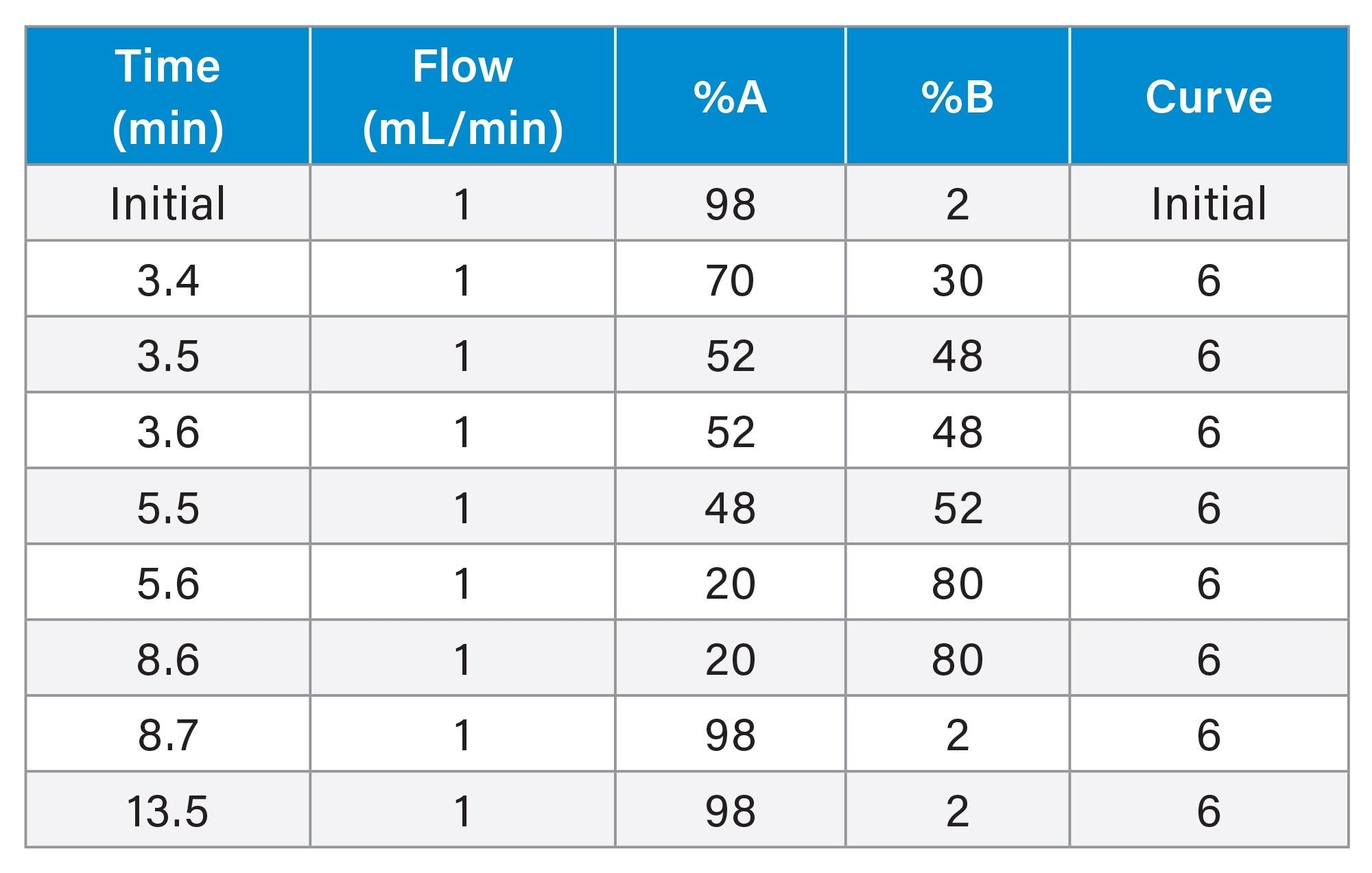
This method was reproducible over the course of 6 injections in the retention and separation of the HIV Drug Mix Standard. At the 260 nm wavelength, the %RSD for area and retention time for the antiretrovirals in the HIV Drug Mix Standard was <1% (Tables 2 and 3). At the 214 nm wavelength, the %RSD for area and retention for enfuvirtide in the HIV Drug Mix Standard was ≤1% (Tables 4 and 5).
The average resolution between Bictegravir (BIC) and Dolutegravir (DOL) was 1.3, however, since BIC and DOL are often not used together in HIV therapy, this should not influence the accurate analysis of combination therapies.5,12 Figures 1a and 1b show overlay chromatograms of the 6 injections at both 260 nm and 214 nm, providing a clear picture of the method’s performance. At 214 nm the enfuvirtide peak had a larger response. Baseline shift was due to the combination of a step gradient and the increase in mobile phase-related background noise, which is commonly seen at lower wavelengths. Further, these chromatograms demonstrate the importance of using the correct wavelength for analysis.
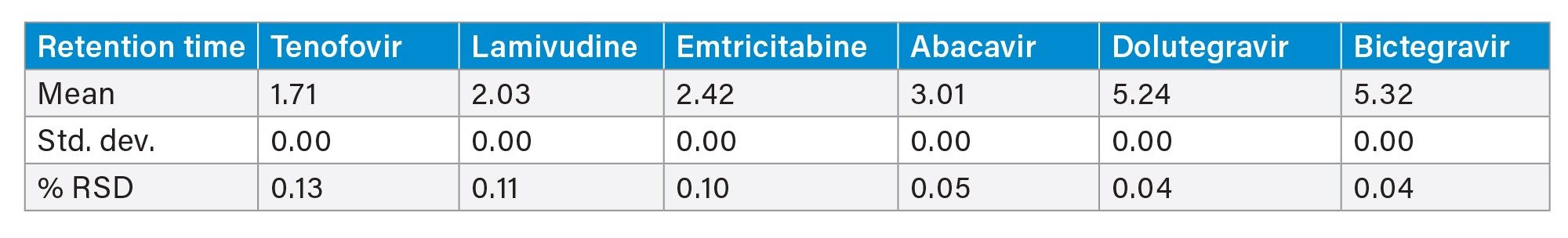
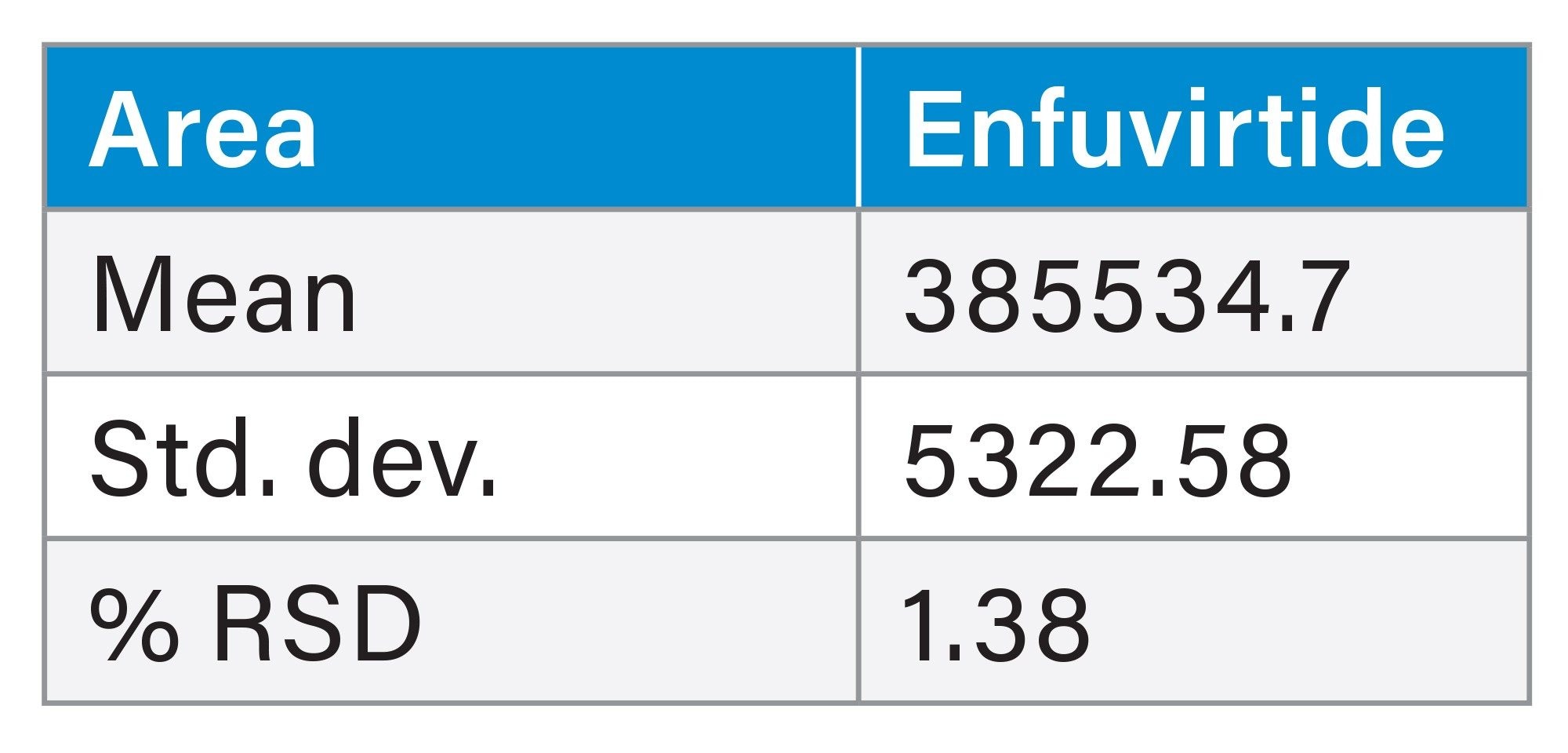
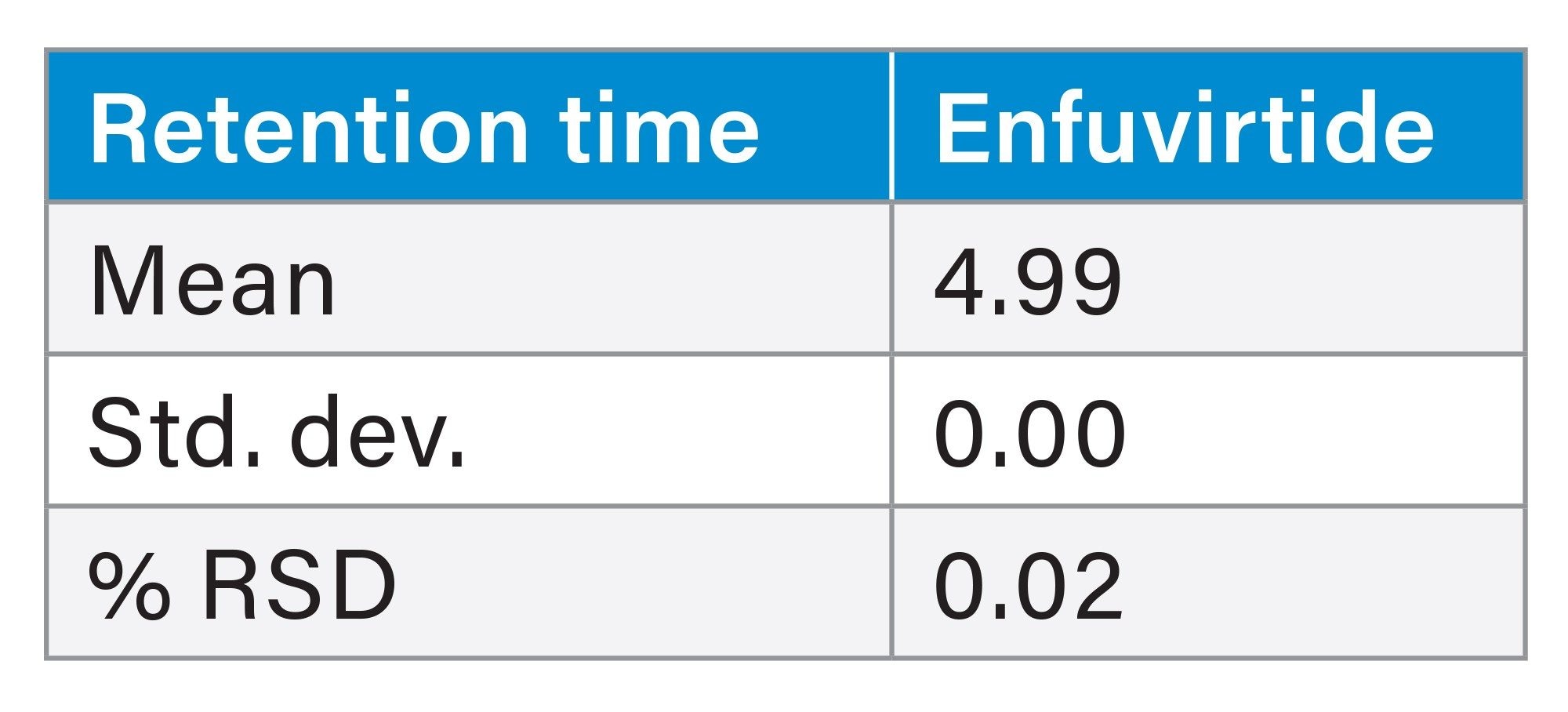
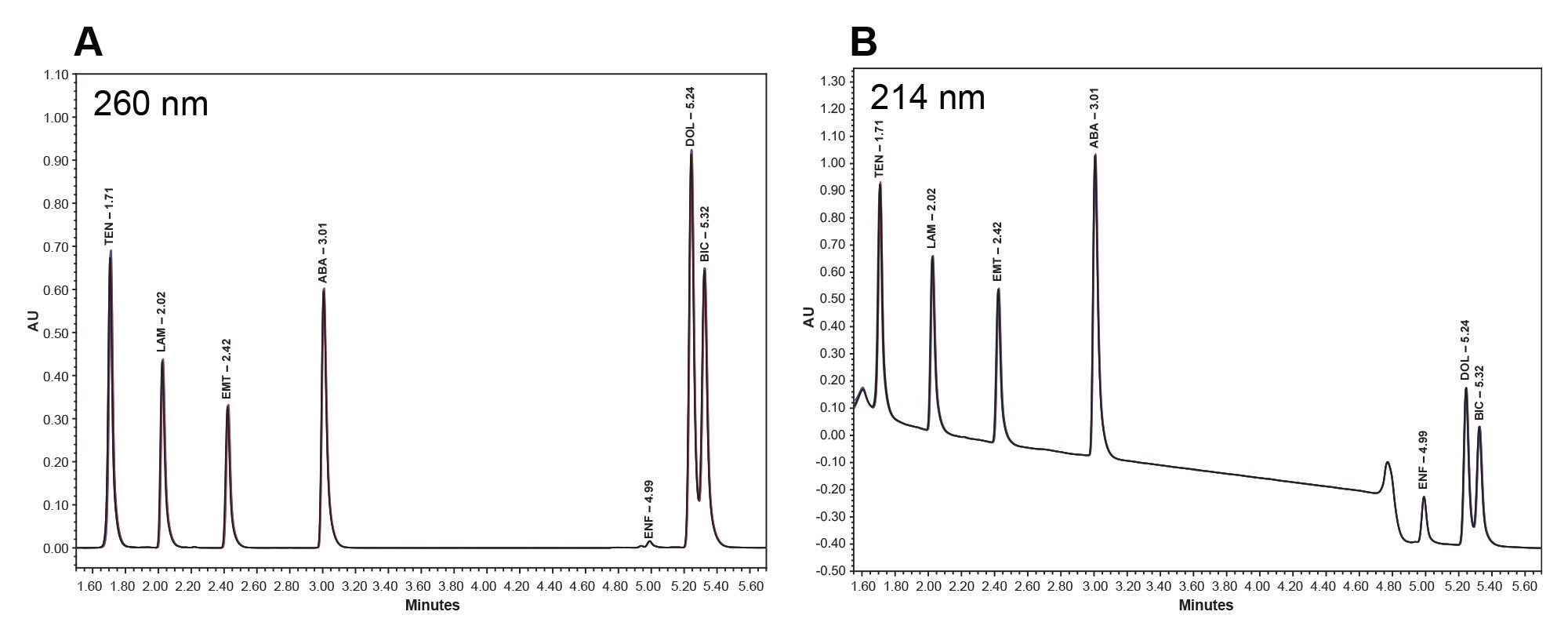
Figure 1b. An overlay chromatogram of the 6 injections of the HIV Drugs Standard at wavelength 214 nm. See Table 1 for listed abbreviations.
Mass Analysis of Enfuviritde:
While enfuvirtide can be detected with a UV detector, mass analysis was performed to aid in the identification of enfuvirtide. Enfuvirtide has a molecular mass of 4492 g/mol.7 While the nominal mass is above the detection limit of the ACQUITY QDa Mass Detector, enfuvirtide has a prominent ion at 1124 m/z, meaning the synthetic peptide can be detected when it has a charge of 4 in relation to its mass to charge ratio (m/z).13,14 Below is a stacked chromatogram depicting the UV and total ion chromatogram (TIC) of the HIV Drugs Standard, as well as the selected extracted ion chromatogram (XIC) for enfuvirtide (Figures 2a through 2c).
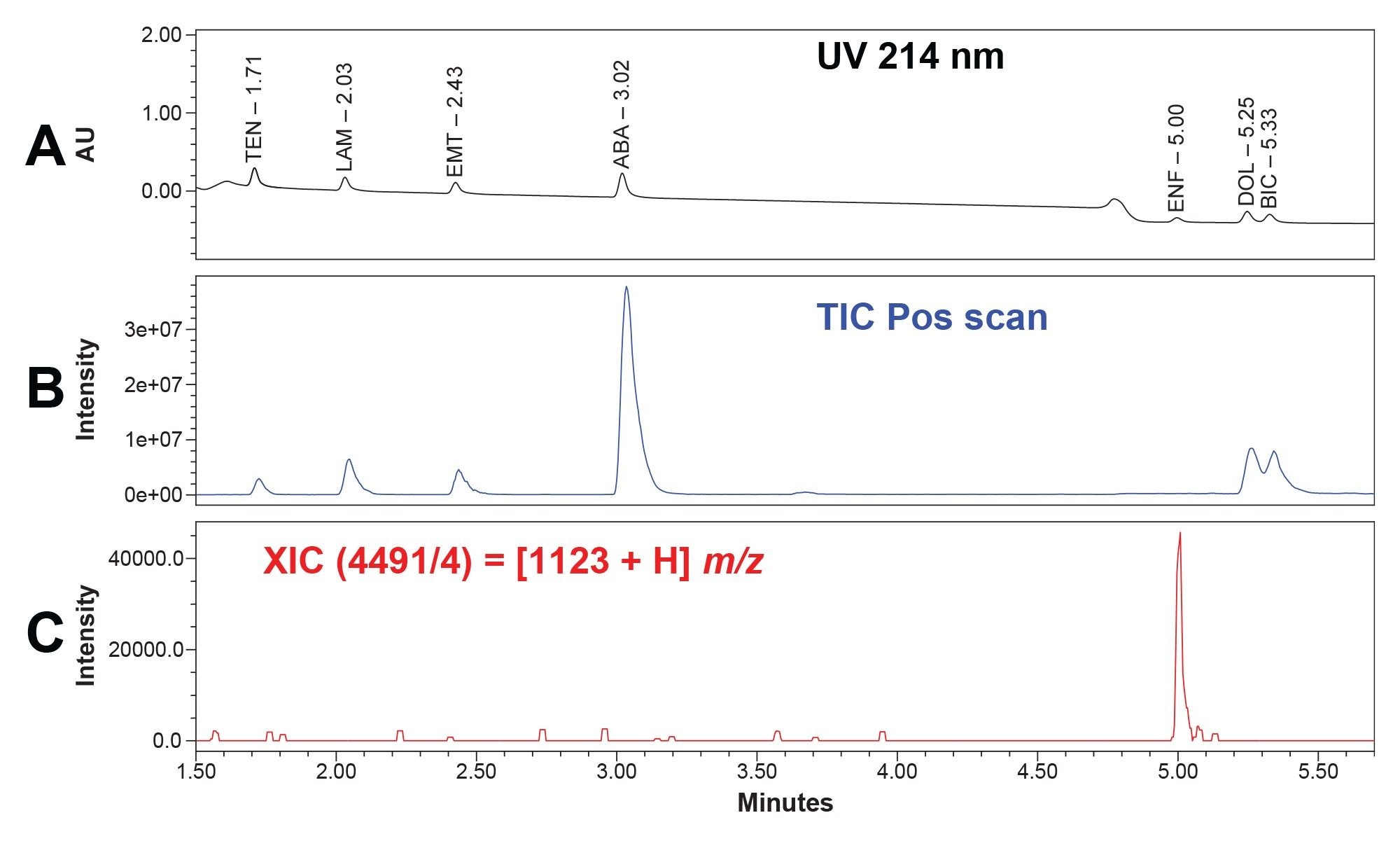
Figure 2b. A TIC chromatogram of the HIV Drugs Standard in the positive ion scan mode, at a 10 μg/mL concentration.
Figure 2c. An XIC chromatogram at the m/z for enfuvirtide demonstrating the ability to detect the peptide using the bench top ACQUITY QDa Mass Detector, at a 10 μg/mL concentration.
Linearity Results:
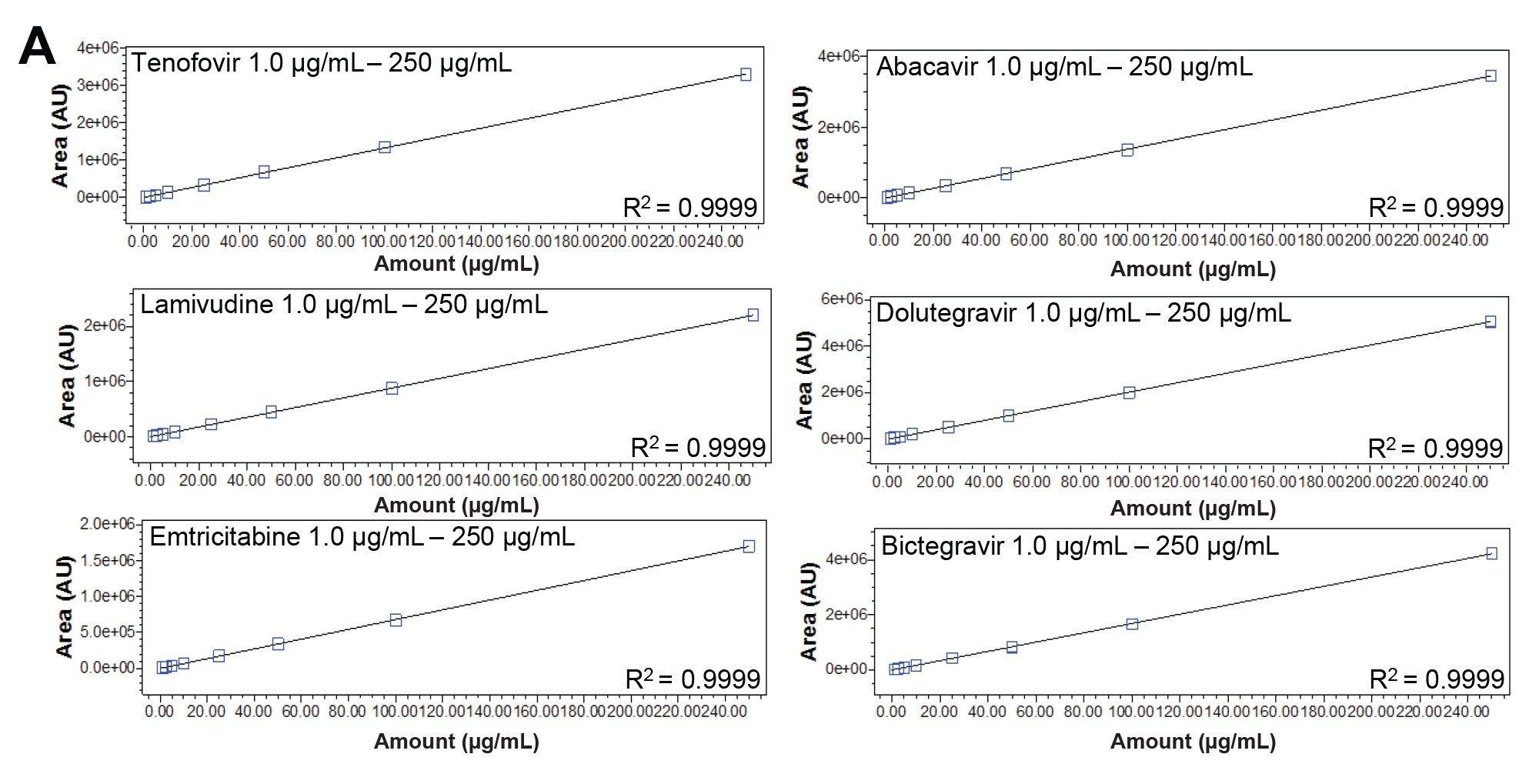
Each of the analytes in the HIV Drug Mix Standard was investigated for their linearity. The linearity of enfuvirtide was examined separately given the substantial differences in response between the synthetic peptide and small molecule antiretroviral drugs. Linearity results for each analyte were calculated using a linear fit through the calibration features in Empower 3. All the linear regression coefficients were ≥0.999. The quantitative capability of this method is clear, suggesting its use in quality control testing (Figures 3a and 3b).

Comparison of Premier to Stainless Steel Results:
This method was used on both a stainless-steel system and a MaxPeak Premier System to highlight the improvements MaxPeak HPS Technology has on synthetic peptide analysis. 10 injections of the enfuvirtide suitability standard were injected onto each of the instrument types. MaxPeak HPS offers more reproducible data sets, as well as a 23% increase in area and 10% increase in height. Below, results for both systems are compared (Table 6, and figures 4a through 4b).
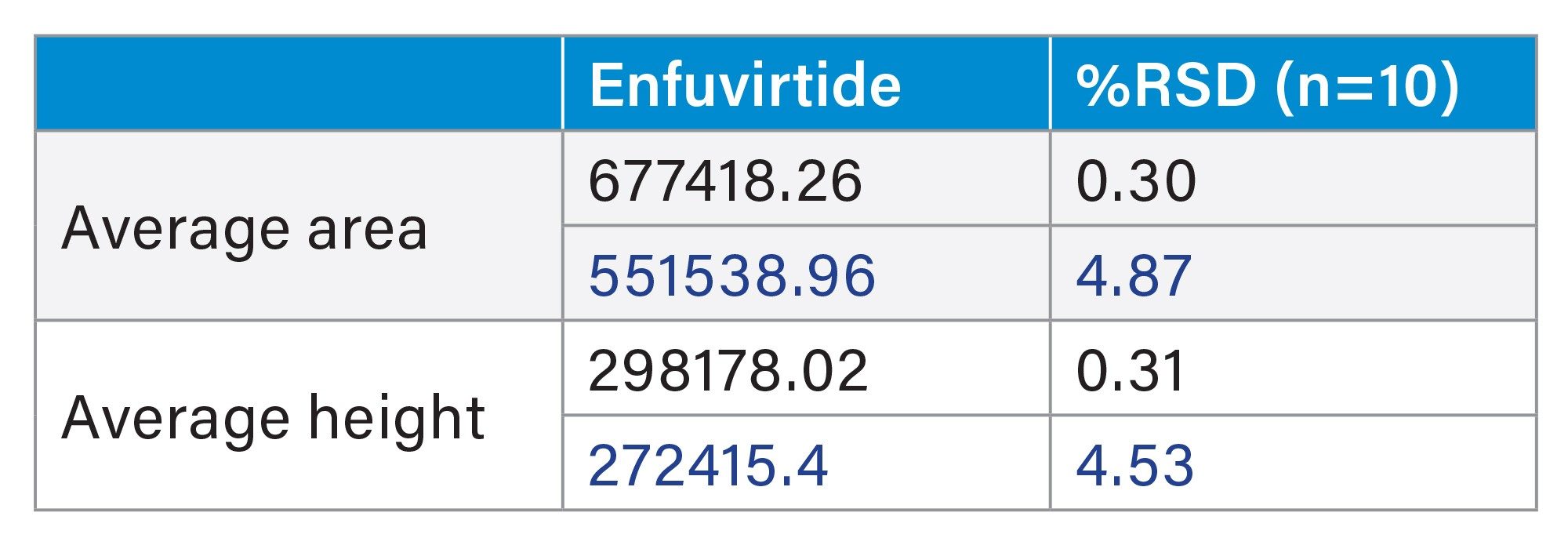
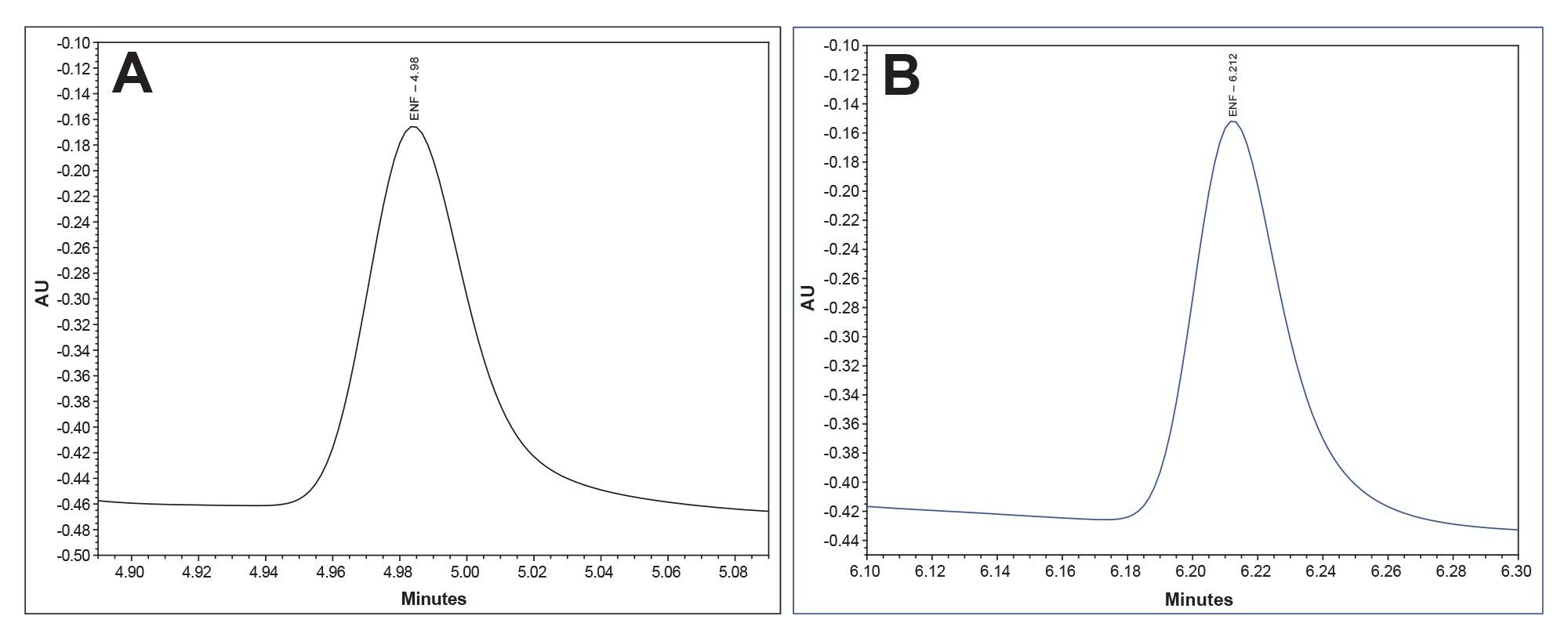
Figure 4b. A representative chromatogram for enfuvirtide in the Enfuvirtide Suitability Standard for the traditional stainless-steel system (Blue).
Limit of Quantitation Results:
Given the increases in height and area, the MaxPeak HPS System offers increases in sensitivity. To measure this, we looked at the LOQ for each system. According to the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH), LOQ can be measured using signal-to-noise ratios.15 The LOQ standard for the Premier MaxPeak HPS System was 3.5 μg/mL. This standard was injected 10 times on each system. The averages signal-to-noise ratio for the Premier MaxPeak HPS system was 14.2, which is above the suggested ratio of 10 for LOQ. For the traditional stainless-steel system, the average signal-to-noise ratio was 2.4, which is below the suggested ratio for limit of detection (LOD). The increases in area and height signals that MaxPeak HPS offers contributed to lower LOQs. (Figures 5a and 5b).
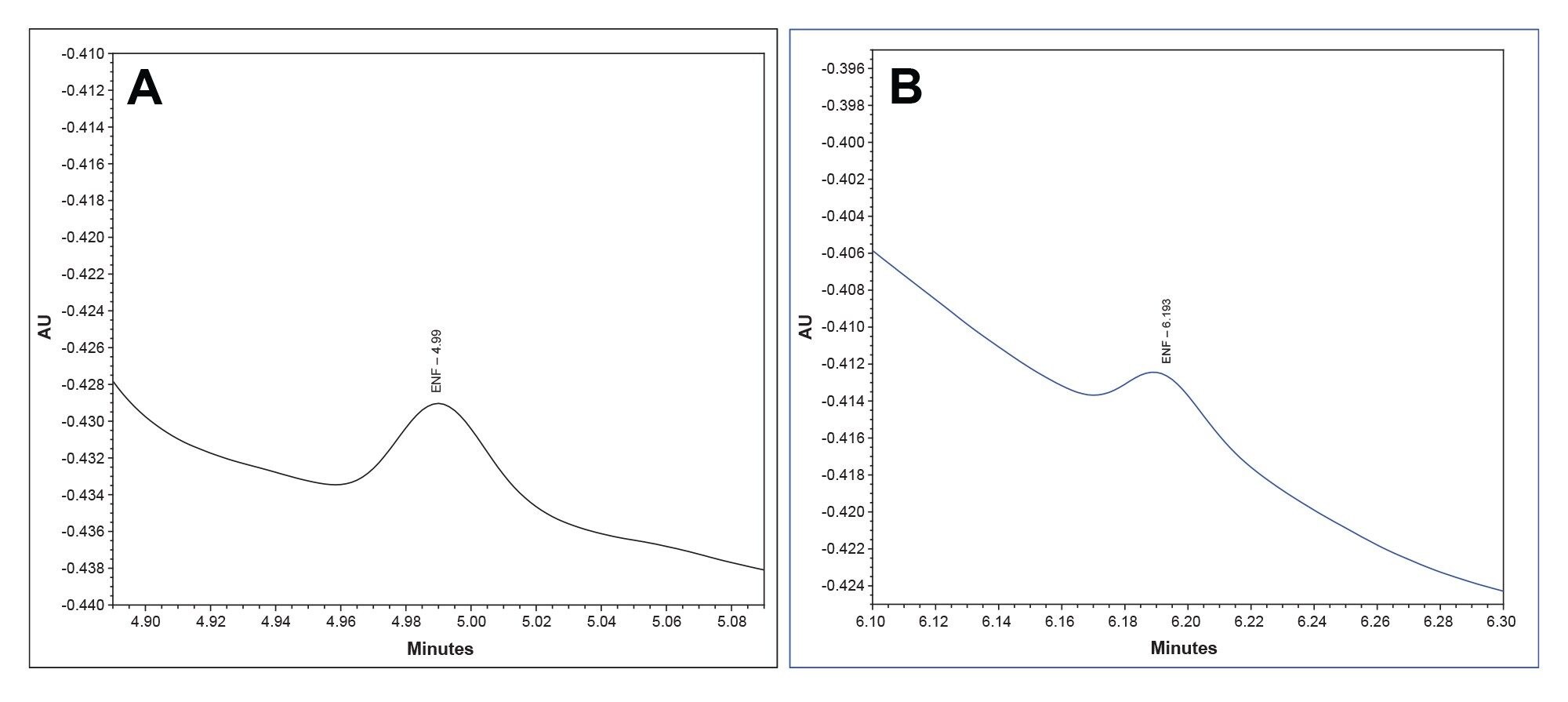
Figure 5b. A representative chromatogram for enfuvirtide at 3.5 μg/mL injected on the traditional stainless-steel system (Blue). The average signal-to-noise ratio was 2.4, over the course of 10 injections.
Conclusion
In this application note, we describe the development of a linear and reproducible method for the analysis of HAARTs and enfuvirtide. Analytes were separated and detected in less than 6 minutes through UV and benchtop MS detection using the ACQUITY QDa Mass Detector. When compared to a traditional stainless-steel system, the MaxPeak HPS solution provided improvements in chromatographic peak area, height, and overall sensitivity for the analysis of enfuvirtide.
References
- UNAIDS. Global HIV & AIDS Statistics — 2020 Fact Sheet. 2021. Available from: https://www.unaids.org/en/resources/fact-sheet
- Centers for Disease Control and Prevention. HIV Basics. 2019. Available from: https://www.cdc.gov/hiv/basics/index.html
- Centers for Disease Control and Prevention. AIDS and Opportunistic Infections. 2019. Available from: https://www.cdc.gov/hiv/basics/livingwithhiv/opportunisticinfections.html
- Pan American Health Organization. Antiretroviral Therapy - PAHO/WHO | 2023. Available from: https://www.paho.org/en/topics/antiretroviral-therapy#:~:text=Antiretroviral%20therapy%20(ART)%20is%20treatment
- National Institute of Health. Appendix B: Drug Characteristics Tables | NIH. 2023 [cited 2023 Aug 11]. Available from: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/drug-characteristics-tables?view=full#table7
- National Institutes of Health. Enfuvirtide - Patient | NIH. 2023. Available from: https://clinicalinfo.hiv.gov/en/drugs/enfuvirtide/patient
- PubChem. Enfuvirtide. National Center for Biotechnology Information. 2023. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Enfuvirtide
- Charbe N, Baldelli S, Cozzi V, Castoldi S, Cattaneo D, Clementi E. Development of an HPLC–UV Assay Method for the Simultaneous Quantification of 9 Antiretroviral Agents in the Plasma of HIV-Infected Patients. Journal of Pharmaceutical Analysis. December 2016. 6(6):396–403.
- D’Avolio A, Siccardi M, Sciandra M, Lorena B, Bonora S, Trentini L, et al. HPLC–MS Method for the Simultaneous Quantification of the New HIV Protease Inhibitor Darunavir, and 11 other Antiretroviral Agents in Plasma of HIV-Infected Patients. Journal of Chromatography B. November 2007. 859(2):234–40.
- Birdsall R, Kellet J, Ippoliti S, Qing Yu Y. Increasing Recovery and Chromatographic Performance of “Acidic” Peptides Using Waters ACQUITY Premier Solution. Waters Application Note. 720007173. 2021.
- Buck MA, Olah TA, Weitzmann CJ, Cooperman BS. Protein Estimation by the Product of Integrated Peak Area and Flow Rate. Analytical Biochemistry. Nov 1989. [cited 2023 Aug 11]. 182(2):295–9. Available from: https://www.sciencedirect.com/science/article/abs/pii/0003269789905976?via%3Dihub
- U.S. Food and Drug Administration. Reviewer Guidance, Validation of Chromatographic Methods. FDA U.S. Food and Drug Administration. 2018. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/reviewer-guidance-validation-chromatographic-methods
- Instrument Specifications ACQUITY QDa Detector. Waters Corporation; 2016 [cited 2023 Aug 11]. Available from: https://www.waters.com/webassets/cms/library/docs/720004799en.pdf
- Chang D, Kolis SJ, Linderholm K, Julian TF, R. Nachi, A.M. Dzerk, et al. Bioanalytical Method Development and Validation for a Large Peptide HIV Fusion Inhibitor (Enfuvirtide, T-20) and Its Metabolite in Human Plasma Using LC–MS/MS. Journal of Pharmaceutical and Biomedical Analysis. 2005 Jul 1;38(3):487–96.
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Ich Harmonised Tripartite Guideline Validation of Analytical Procedures: Text and Methodology Q2(R1). ICH. November 2005 . Available from: https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf
720008072, October 2023