Quantification of Semaglutide in Human Plasma Using Xevo™ TQ-XS Mass Spectrometer: A Highly Sensitive and Reliable Analytical Method
Abstract
In this study, we present the development and validation of a highly sensitive and robust analytical method for the accurate quantification of Semaglutide in human plasma using Xevo TQ-XS tandem quadrupole Mass Spectrometer coupled with ACQUITY™ Premier UPLC™. The LC-MS/MS method exhibits optimized parameters for mass detection and chromatographic separation using the ACQUITY Premier Peptide BEH™ C18 Column. The incorporation of Difluoroacetic acid (DFA) as an ion pairing reagent improves peak shape and sensitivity for charged analytes, resulting in sharper peaks and enhanced ionization efficiency. The method's validation confirms its excellent selectivity, sensitivity, and reproducibility, with linearity assessed over the clinically relevant concentration range (0.1 ng/mL to 20 ng/mL). Precision and Accuracy (P & A) evaluation using Quality Control (QC) samples demonstrates satisfactory results, with % Accuracy falling within an acceptable range and Coefficient of Variation (CV) values below 8% RSD. The developed method holds significant promise for advancing research and clinical applications in diabetes management, providing a valuable tool for accurate Semaglutide quantification, and contributing to the progress of peptide analysis in bioanalytical sciences.
Benefits
- Precise and accurate determination: The method allows for the precise and accurate measurement of Semaglutide at the Lower Limit of Quantitation (LLOQ) as low as 0.1 ng/mL
- Highly specific and selective sample preparation: The use of SPE (solid-phase extraction) ensures high specificity and selectivity during the sample preparation process, concentrate analyte from complex sample matrices before their analysis leading to reliable results
- Chromatographic separation: The premier BEH Peptide Column is employed for chromatographic separation, designed to reduce nonspecific binding of compounds that interact with metals, thus providing enhanced sensitivity and improved peak shape
- Use of ACQUITY Premier UPLC: The system incorporates MaxPeak™ surfaces, which reduce surface interactions and adsorption, leading to better analyte recovery, reproducibility, and sensitivity minimizing the carryover effects
- Use of QuanRecovery™ Polypropylene vials: These vials are designed to minimize analyte adsorption, improve recovery rates, and ensure the reproducibility of sample analysis. It reduces nonspecific binding of analytes, leading to improved recovery rates during sample extraction and analysis
Introduction
Semaglutide, a glucagon-like peptide-1 (GLP-1) analog, has emerged as a promising therapeutic option for the management of type 2 diabetes mellitus and obesity. It is a newly developed GLP-1 receptor agonist, structurally similar to liraglutide but modified for a longer half-life.1 In 2020, it ranked 129th among commonly prescribed medications for T2D in the United States, with over four million prescriptions. T2D affects nearly 9% of adults worldwide, with T2D accounting for about 90% of diabetes cases.2–3 Effective glycemic control is vital in managing T2D and reducing associated complications. Semaglutide, alike human incretin GLP-1, boosts insulin secretion and blood sugar disposal, leading to improved glycemic control. Thus, known to be an adjunct to diet and exercise in adults with type 2 diabetes.4 Its once-weekly dosing regimen and potent glycemic control effects have garnered considerable attention in clinical practice.5–6 To ensure safe and effective clinical administration of Semaglutide, accurate and reliable quantification of this peptide in human plasma is crucial. In the present study, the development and validation of a highly sensitive and robust analytical method for the precise quantification of Semaglutide in human plasma is presented using the Xevo TQ-XS tandem quadrupole Mass Spectrometer. The analytical method's optimization was driven by the need for enhanced sensitivity, reliable reproducibility, and minimized matrix interferences in the complex plasma samples.
The validation process of the method adhered strictly to international regulatory guidelines, ensuring the method's precision, accuracy, and selectivity. Calibration standards were prepared to cover the clinically relevant concentration range, and quality control samples were incorporated to assess method robustness.
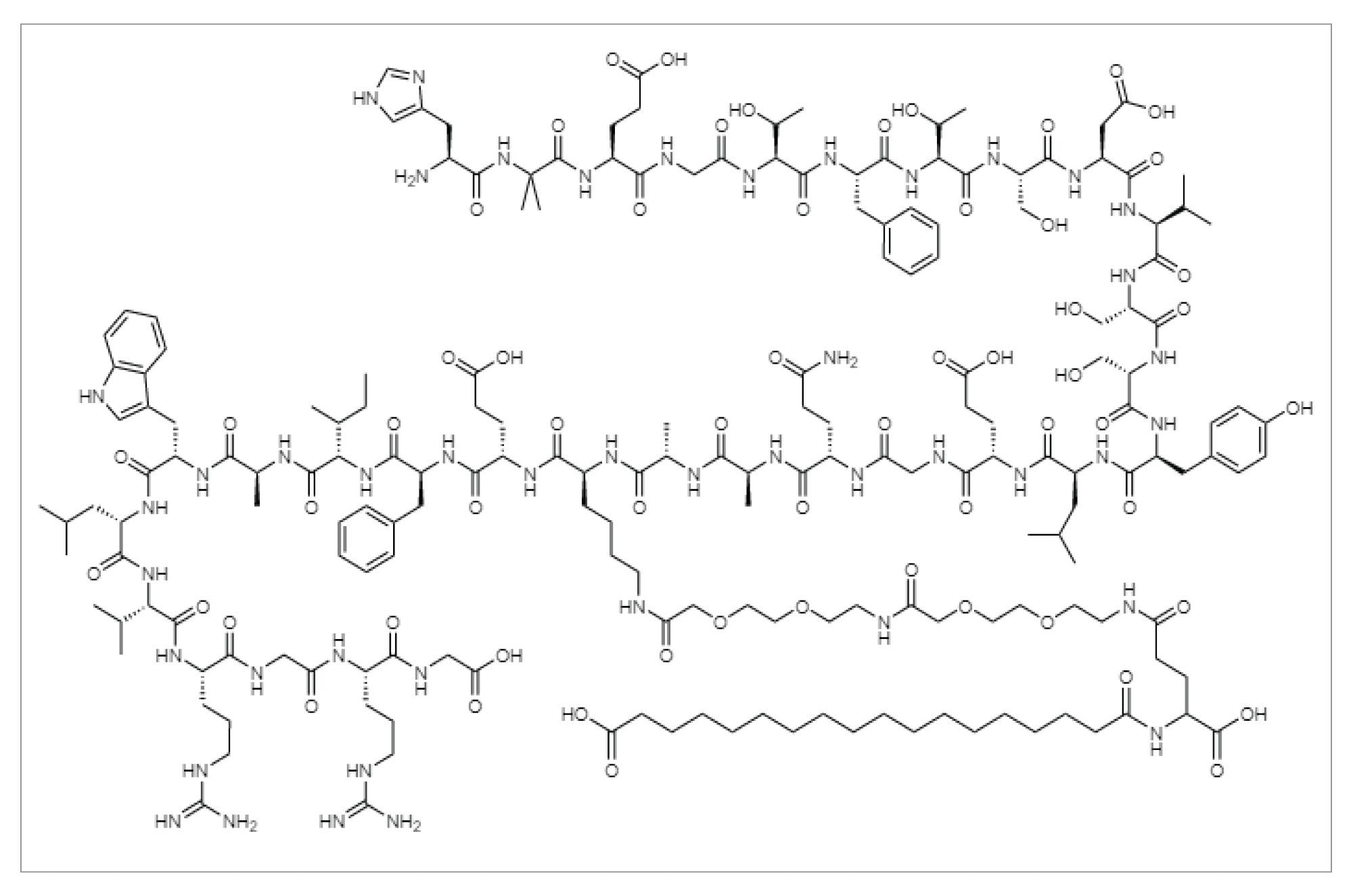
Experimental
For this study, standard and spiking solutions were diluted using a mixture of Water and Acetonitrile in a 1:1 ratio, containing 2% formic acid. To create the calibration curve spanning from 0.1 to 20 ng/mL, test portions of human plasma were spiked with known quantities of Semaglutide standard. Additionally, quality control (QC) samples were prepared with Semaglutide spiked at four different levels: 0.1 ng/mL (Lower Limit QC), 0.5 ng/mL (Low QC), 5 ng/mL (Mid QC), and 20 ng/mL (High QC). Notably, this experiment did not involve the use of an internal standard.
Sample Preparation
To begin sample preparation, 500 µL of human plasma was taken and treated with an equal amount of acetonitrile. After thorough vortexing for one minute, the samples underwent centrifugation at 15000 rpm at 10 °C for 5 minutes.
Method Conditions
LC Conditions
|
LC system: |
ACQUITY I-Class Premier UPLC with FTN SM |
|
Vials: |
Quan Recovery, MaxPeak 12 x 32 mm PP 300 µl screw cap vials (p/n: 186009186) |
|
Columns: |
ACQUITY Premier Peptide BEH C18, 300 Å, 1.7 µm, 2.1 x 100 mm (p/n: 186009494) |
|
Column temperature: |
70 °C |
|
Sample temperature: |
10 °C |
|
Injection volume: |
15 µL |
|
Mobile phase A: |
Water with 0.4% formic acid |
|
Mobile phase B: |
Acetonitrile with 0.1% DFA |
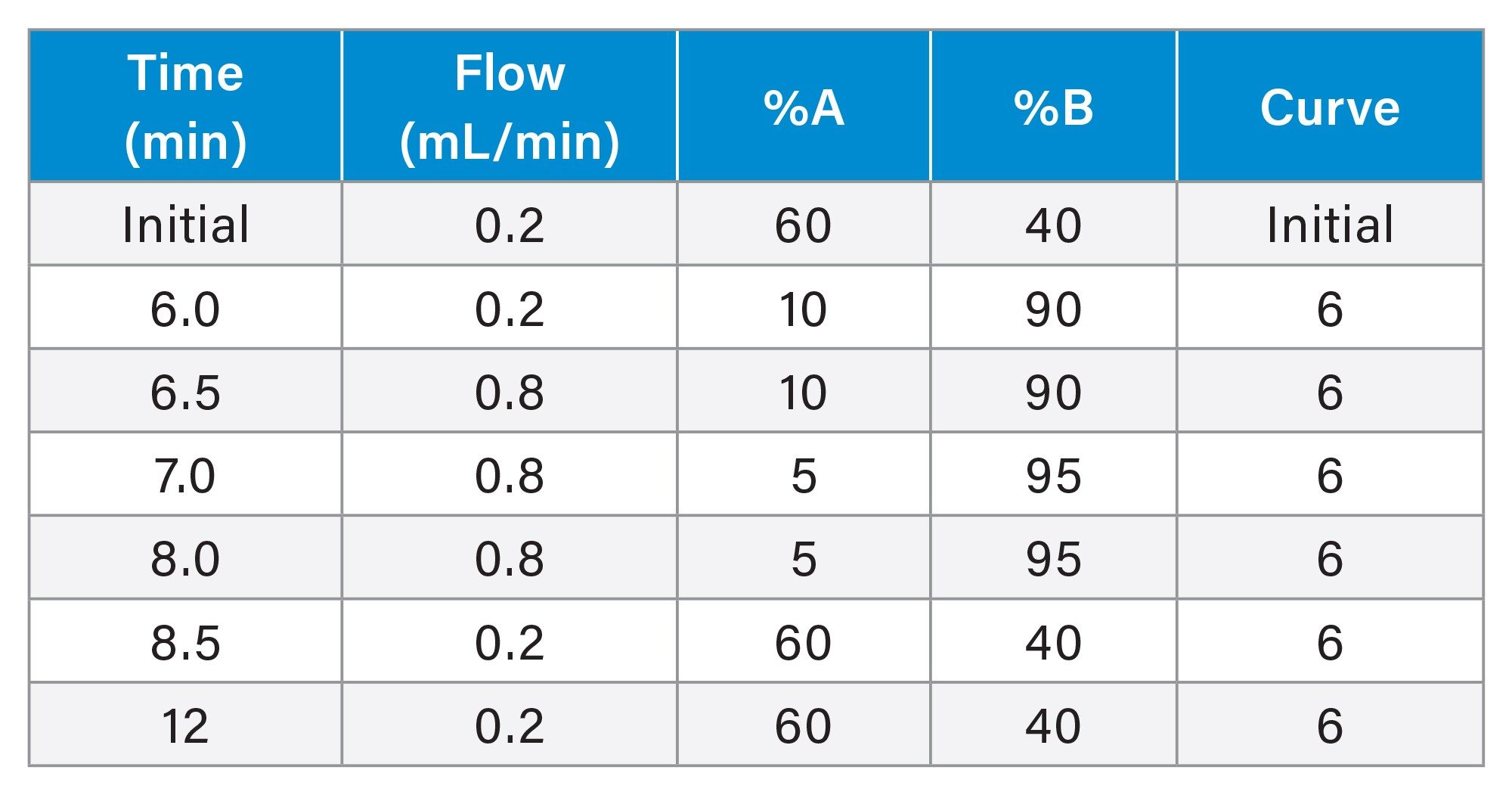
Gradient Table
MS Conditions
|
MS system: |
Xevo TQ-XS |
|
Ionization mode: |
Electrospray (positive ion mode) |
|
Capillary voltage: |
3.0 kV |
|
Source temperature: |
150 °C |
|
Desolvation temperature: |
600 °C |
|
Desolvation gas flow: |
1100 L/hr |
|
Cone gas flow: |
300 L/hr |
|
Cone voltage: |
50 V |
Table 2. Optimized MS parameters for Semaglutide.
MRM Method (quantitative transition given in bold)
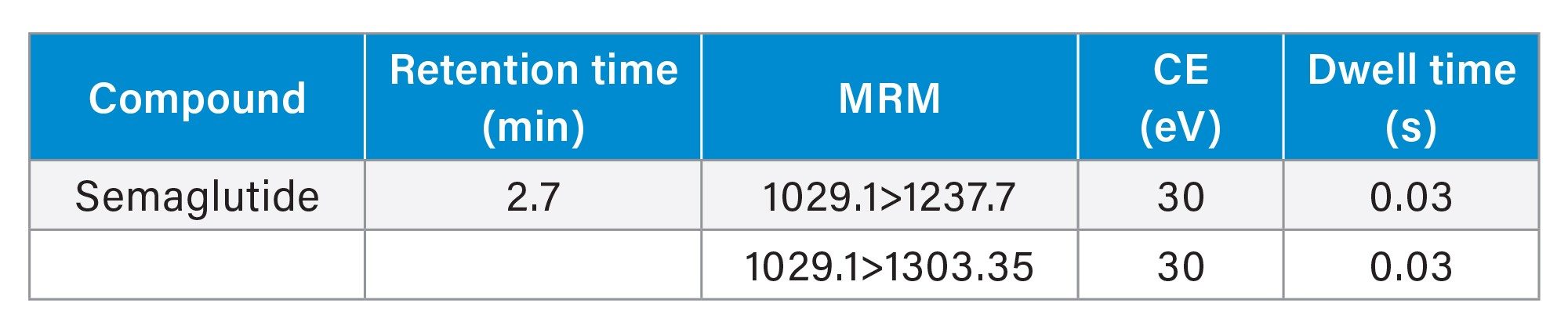
The dwell times were set automatically using the auto dwell function to give a minimum of 20 data points across each peak.
Data Management
|
MS software: |
Masslynx™ v4.2 |
|
Informatics: |
Targetlynx™ XS |
Results and Discussion
In this work, a complete sample preparation and UPLC LC-MS/MS method for accurate quantification of Semaglutide from plasma was developed. The method includes a mixed-mode SPE extraction using Oasis 1 cc (30 mg) Cartridge.
Quantification was performed using the Xevo TQ-XS Tandem Quadrupole MS (ESI+). The tuning process is critical to ensure that the instrument can efficiently detect and quantify the desired ions, providing precise and accurate results for the identification and quantification of Semaglutide in samples. Semaglutide, like many other peptides and proteins, exhibit multiple charging in electrospray Mass Spectrometry due to its structure and composition. This phenomenon is common for large biomolecules, (e.g., peptides and proteins) and is a result of the ionization process used in Mass Spectrometry, particularly electrospray ionization (ESI). Because Semaglutide is a peptide with multiple basic amino acid residues (e.g., lysine and arginine), it can attract multiple protons during the ionization process. Each protonation results in a positively charged ion. As a result, several different multiply charged ions of Semaglutide may be observed in the mass spectrum as depicted in Figure 3. Notably, the ion with the highest intensity, m/z 1029, representing a 4+ ion, was chosen for quantification purposes.
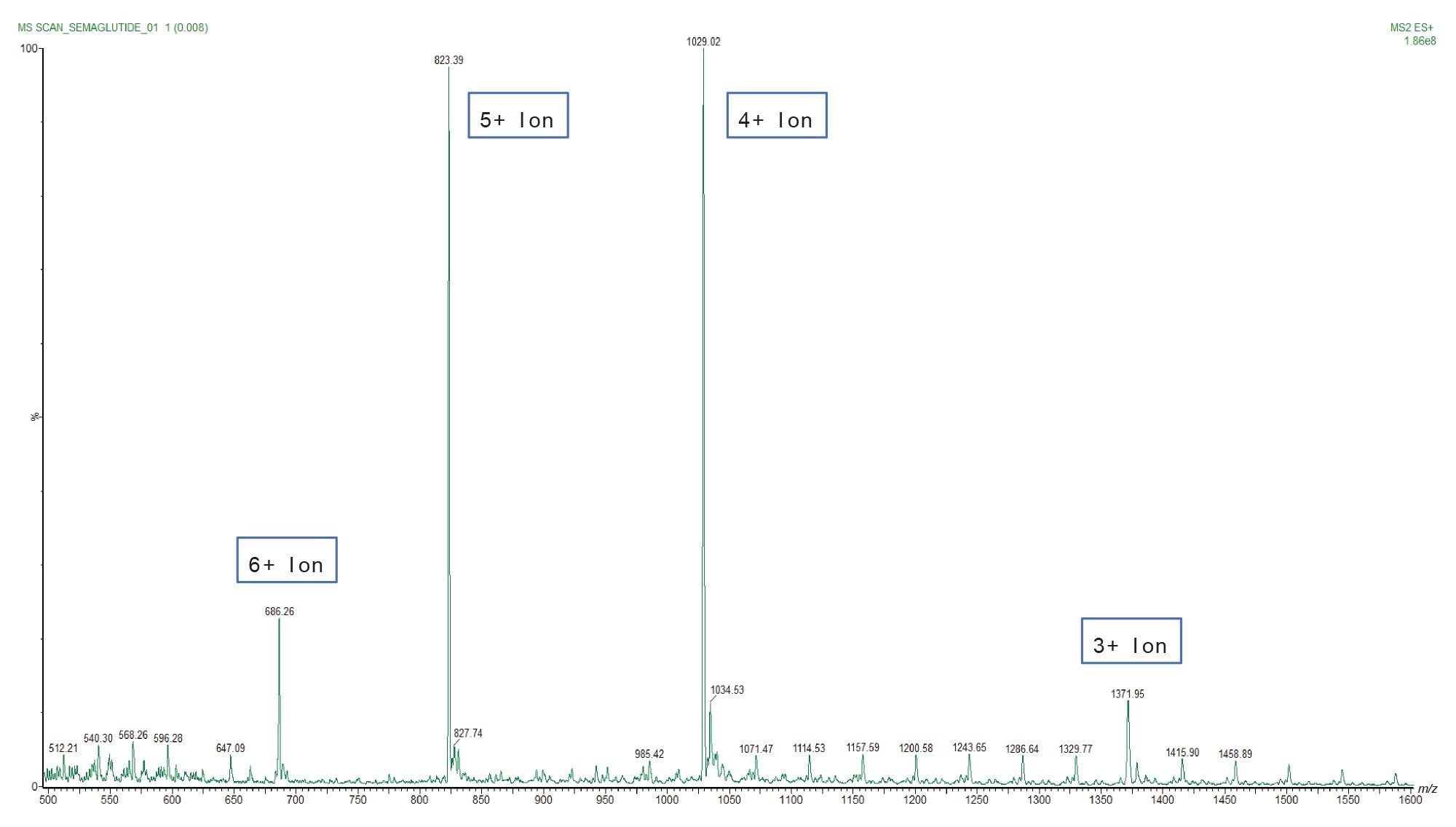
Optimized MS parameters and MRM transitions are outlined in the experimental section, Table 2 and Table 3 respectively, mentioned in the above comment.
Chromatographic separation was performed by using ACQUITY Premier Peptide BEH C18, 300 Å, 1.7 µm, 2.1 x 100 mm) and ACQUITY I-Class Premier UPLC chromatography System. ACQUITY Premier columns and chromatography systems are made with hardware technology designed to reduce nonspecific binding of compounds that interact with metals, thus providing enhanced sensitivity and improved chromatographic peak shape. This column designed specifically for peptide separations as it offers good retention and selectivity by providing hydrophobic interactions with the peptides. The column provided high resolution, sensitivity, and reproducibility for Semaglutide separation from matrix.
In addition to this, Difluoro acetic acid (DFA) was used in mobile phase as an ion pairing reagent to improve the peak shape of charged analytes. The formation of ion pairs with DFA increased the ionization efficiency leading to improved sensitivity in the mass spectrometer DFA also helped reducing peak tailing by promoting protonation and preventing unwanted secondary interactions with the stationary phase. (Ref.7)
The developed method demonstrated excellent selectivity, sensitivity, and reproducibility. Figure 4 shows a typical chromatogram of the matrix blank and a spiked sample at the Lower Limit of Quantitation (LLOQ) of 0.1 ng/mL, where the analyte appeared as an excellent symmetrical peak at 2.71 minutes, free from matrix interferences. Additionally, the Signal-to-Noise (S/N) ratio at LLOQ (0.1 ng/mL) exceeded 30 without smoothening.

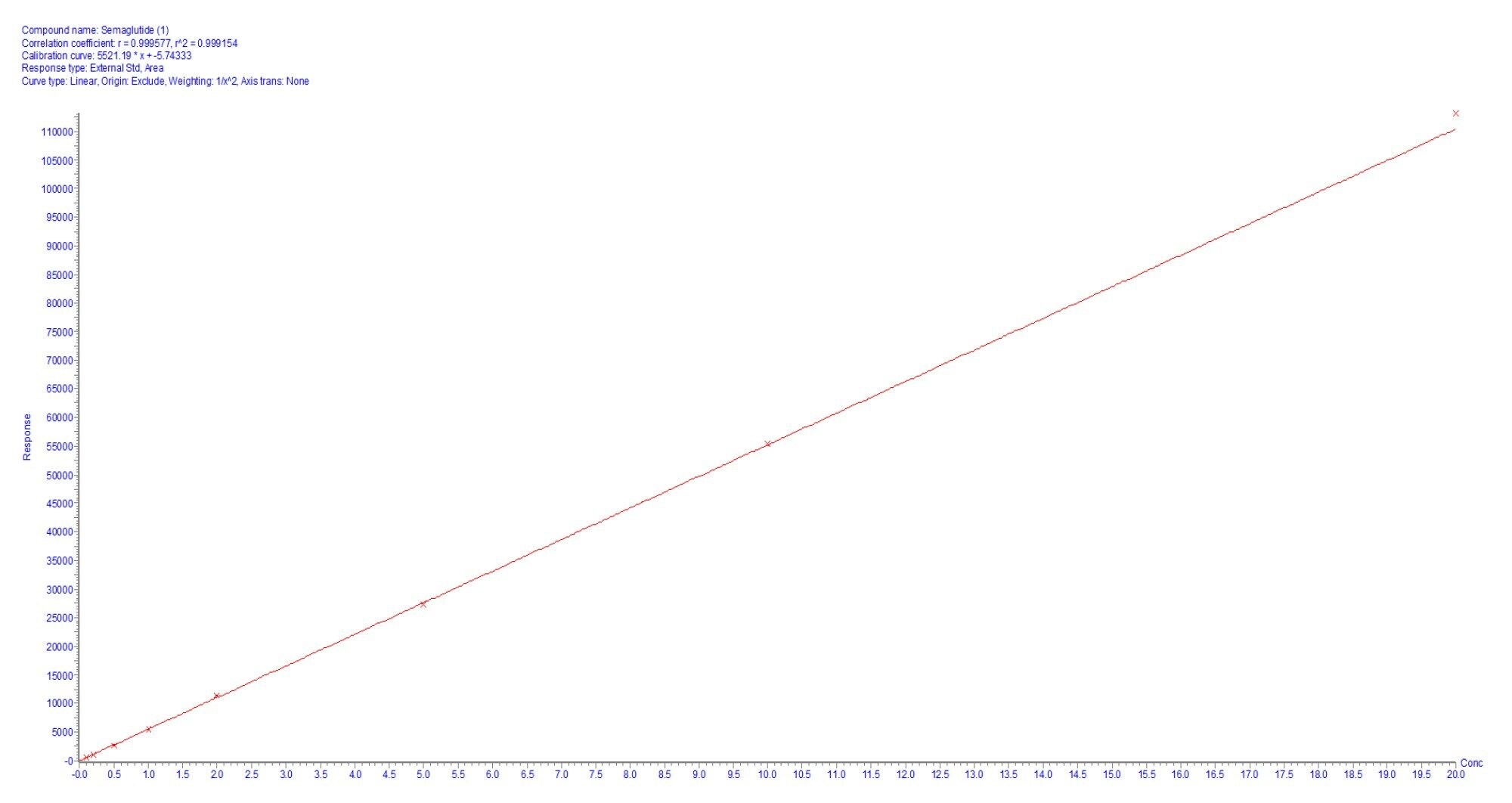
Linearity was assessed over the concentration range of 0.1 ng/mL to 20 ng/mL, yielding an excellent correlation coefficient of 0.999. A representative calibration curve within this range is depicted in Figure 6.
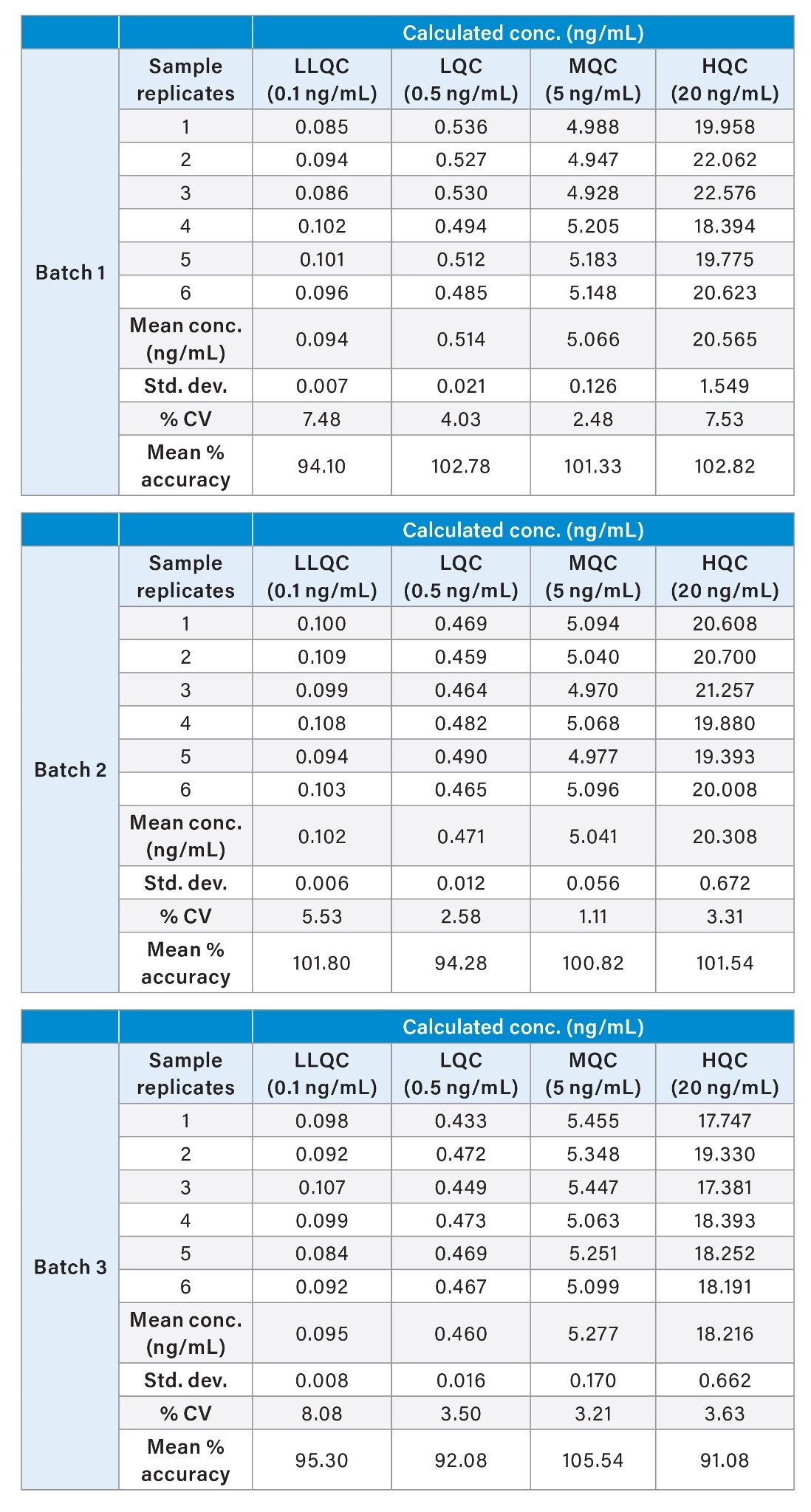
For Precision and Accuracy (P & A) evaluation, QC samples were spiked at four different levels: 0.1 ng (LLOQQC), 0.5 ng (LQC), 5 ng (MQC), and 20 ng (HQC). Three separate P & A batches were conducted on different occasions by different chemists. The results are summarized in Table 4.
The % Accuracy in pre-spiked plasma samples at all QC levels fell within an acceptable range at respective QC levels. The Coefficient of Variation at each QC level in all three batches was below 8% RSD. Moreover, the mean % accuracy at the four QC levels in all three batches ranged from 92% to 106%, meeting the acceptance criteria outlined in the bioanalytical validation guidelines.
Conclusion
In conclusion, a comprehensive and robust sample preparation and UPLC LC-MS/MS method for the accurate quantification of Semaglutide from plasma is developed. The Xevo TQ-XS Tandem Quadrupole MS (ESI+) provided sensitive and reliable quantification of Semaglutide, with the tuning process critical to achieving precise and accurate results.
Employing ACQUITY Premier UPLC Chromatography System and Column with the MaxPeak surfaces, effectively mitigating surface interactions and analyte adsorption. This integration lead to enhanced analyte recovery, improved reproducibility, heightened sensitivity, and a reduction in carryover effects.
Chromatographic separation using the ACQUITY Premier Peptide BEH C18 Column offered excellent retention, selectivity, and sensitivity, ensuring efficient resolution of complex peptide mixtures. The inclusion of Difluoroacetic acid (DFA) as an ion pairing reagent further improved peak shape and sensitivity for charged analytes in reversed phase LC, resulting in sharper peaks and increased ionization efficiency in the Mass Spectrometer.
The validation results demonstrated the method's excellence, with excellent linearity, precision, and accuracy across the concentration range from 0.1 ng/mL to 20 ng/mL. The % Accuracy and Coefficient of Variation at different QC levels met the acceptance criteria, ensuring reliable and reproducible quantification of Semaglutide in human plasma.
This developed method holds significant promise for advancing research and clinical applications in the field of diabetes management. The robustness and sensitivity of method provide a valuable tool for accurate Semaglutide quantification, supporting safe and effective therapeutic use and contributing to the advancement of peptide analysis in bioanalytical sciences.
References
- 14 December 2017 EMA/CHMP/715701/2017 Committee for Medicinal Products for Human Use (CHMP).
- "The Top 300 of 2020". ClinCalc. Archived from the original on 18 March 2020. Retrieved 7 October 2022.
- "Semaglutide – Drug Usage Statistics". ClinCalc. Archived from the original on 8 October 2022. Retrieved 7 October 2022.
- "Ozempic- semaglutide injection, solution". DailyMed. Archived from the original on 5 June 2021. Retrieved 5 June 2021.
- Goldenberg RM, Steen O. "Semaglutide: Review and Place in Therapy for Adults With Type 2 Diabetes". Canadian Journal of Diabetes. (March 2019) 43 (2): 136–145. doi:10.1016/j.jcjd.2018.05.008. PMID 30195966.
- Kapitza C, Nosek L, Jensen L, Hartvig H, Jensen CB, Flint A. "Semaglutide, a once-weekly human GLP-1 analog, does not reduce the bioavailability of the combined oral contraceptive, ethinylestradiol/levonorgestrel". Journal of Clinical Pharmacology. (May 2015) 55 (5): 497–504. doi:10.1002/jcph.443. PMC 4418331. PMID 25475122.
- Melvin Blaze, Thomas H. Walter. Difluoroacetic Acid as a Mobile Phase Modifier for LC-MS Analysis of Small Molecules. Waters application note 720006776 2020.
720008160, December 2023