Column Selection for RPLC-UV Impurity Analysis of Fatty Acid Modified GLP-1 Receptor Agonists
Abstract
Here, we screen four column chemistries for the RPLC-UV impurity analyses of liraglutide and semaglutide. The columns and chromatographic conditions presented here provide direction and analyte-specific tailoring for the development of RPLC-UV methods for fatty acid modified GLP-1 receptor agonists (GLP-1 RAs).
Benefits
- Comparison of five columns for RPLC-UV impurity analysis of fatty acid modified GLP-1 RAs
- Recommended columns and chromatographic conditions for lipopeptide impurity analysis method development
- MS identification of impurities with abundances as low as 0.1% despite ion suppression from TFA
Introduction
Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are a class of biotherapeutic peptide-like drugs developed to treat Type 2 diabetes. The recent FDA approval of multiple GLP-1 RAs for weight loss has led to rapid increase in their demand and use.1 The prevalence of these drugs prompts a need for robust chromatographic methods to screen for impurities that may impact their safety and efficacy.
GLP-1 RAs consist of a wide range of chemical structures including unmodified synthetic peptides (e.g. exenatide), peptide-modified Fc fragments of human IgG4 (e.g. dulaglutide), and fatty-acid modified peptides (e.g. liraglutide, semaglutide, tirzepatide). Waters recently published a single RPLC-UV/MS method for the analysis of a series of GLP-1 RAs using a systematic protocol for method development.2 This method is a valuable starting point, but tailoring the column chemistry and chromatographic conditions may be necessary to effectively separate GLP-1 RA drug products from their impurities due to the variety and complexity of GLP-1 RA chemical structures.
Here, we screen four column chemistries for the RPLC-UV analysis of two fatty acid modified GLP-1 RAs, liraglutide and semaglutide. We demonstrate that minor differences in the chemical structure of liraglutide and semaglutide strongly impact their reversed phase impurity separation and suggest that column selection for RPLC-UV impurity analysis of fatty acid modified GLP-1 RAs should be specifically tailored to the analyte of interest. The presented methodology aims to provide additional direction and analyte-specific tailoring for the development of RPLC-TUV methods for fatty acid modified GLP-1 RAs. Additionally, we show that the reported method enables MS identification of impurities with abundances as low as 0.1% despite ion suppression from TFA.
Experimental
Sample Description
Liraglutide (research grade, AA Blocks lot no. 2885387) was reconstituted at 6 mg/mL in a 3 mM disodium phosphate buffer containing 20 mM phenol and diluted to 0.5 mg/mL with water prior to injection. Semaglutide (Ozempic®) was analyzed past expiry and diluted to 0.5 mg/mL with water prior to injection.
Method Conditions
|
LC system: |
ACQUITY™ UPLC™ I-Class PLUS |
|
Columns: |
CORTECS™ Premier C18+, 1.6 µm, 2.1 x 150 mm (p/n: 186010429) CORTECS Premier C18+, 2.7 µm, 2.1 x 150 mm (p/n: 186010457) ACQUITY Premier Peptide CSH™ C18, 130 Å, 1.7 µm, 2.1 x 150 mm (p/n: 186009489) ACQUITY Premier CSH Phenyl-Hexyl, 1.7 µm, 2.1 x 150 mm (p/n: 186009476) ACQUITY Premier BEH™ C8, 1.7 µm, 2.1 x 150 mm (p/n: 186010358) |
|
Column temperature: |
60 °C |
|
Sample temperature: |
6 °C |
|
Injection volume: |
6 µL |
|
Mobile phase A: |
0.1% TFA in H2O |
|
Mobile phase B: |
0.1% TFA in ACN |
Gradient Table
RDa Detector Settings
|
Mass range: |
50–2000 m/z |
|
Mode: |
ESI+ Full Scan |
|
Sample rate: |
1 Hz |
|
Capillary voltage: |
1.2 kV |
|
Cone voltage: |
20 V |
|
Desolvation temperature: |
350 °C |
|
Data management: |
waters_connect™ |
Results and Discussion
Method Considerations and Column Selection
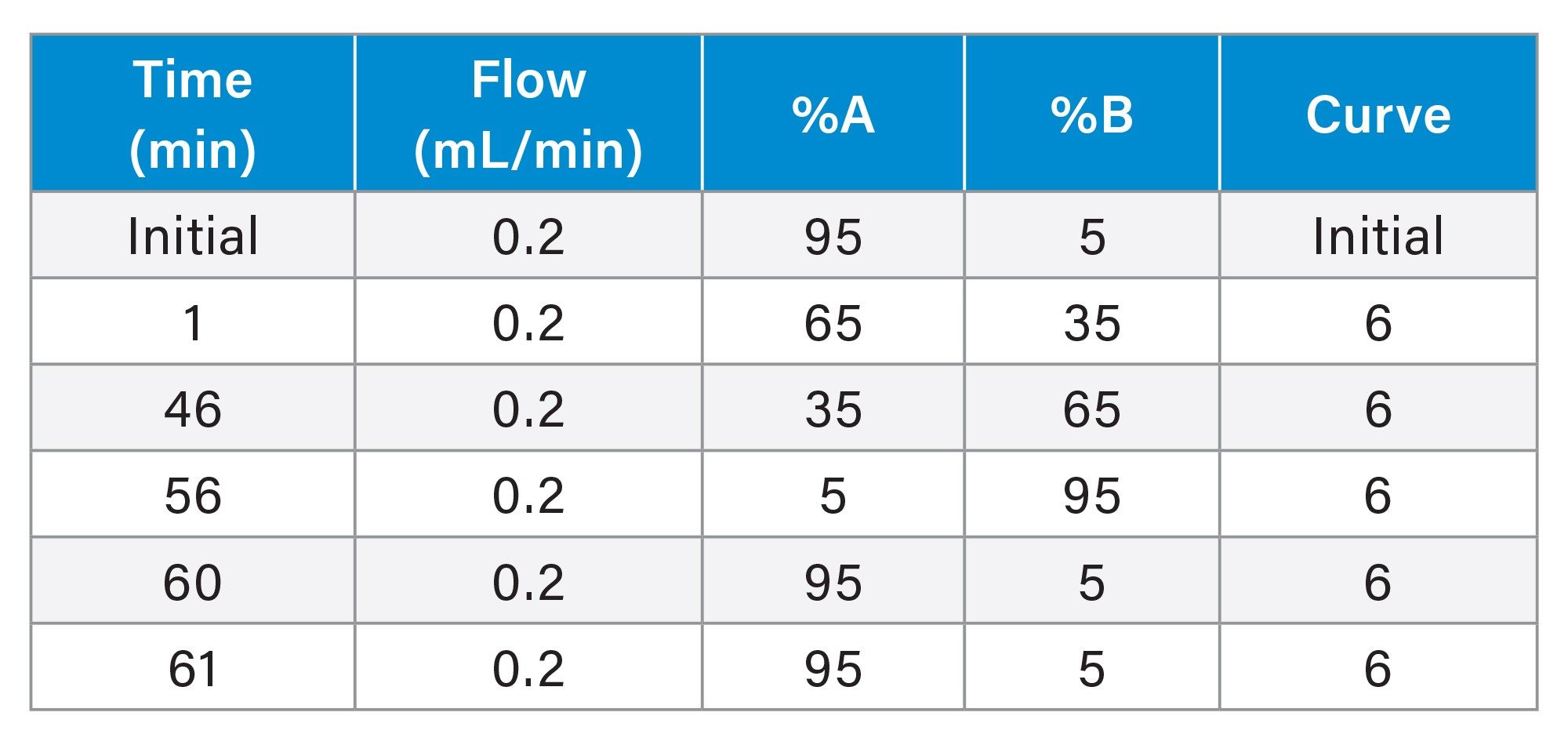
Liraglutide and semaglutide are highly retentive analytes under reversed phase conditions; columns with low retentivity were therefore evaluated for this work. The selected columns are listed in Table 1. Each column is packed in High Performance Surface Technology hardware (i.e. MaxPeak™ Premier) to reduce unwanted analyte/surface interactions. A single method was used to assess each column to simplify column-to-column comparison. TFA, a strong acid and ion-pairing reagent, was selected as the mobile phase additive due to its baseline noise and peak capacity advantages. Finally, a shallow gradient (1.1%/CV for fully porous columns and 1.7%/CV for solid core columns, CV=column volume) was used to achieve high resolution of structurally similar impurities.
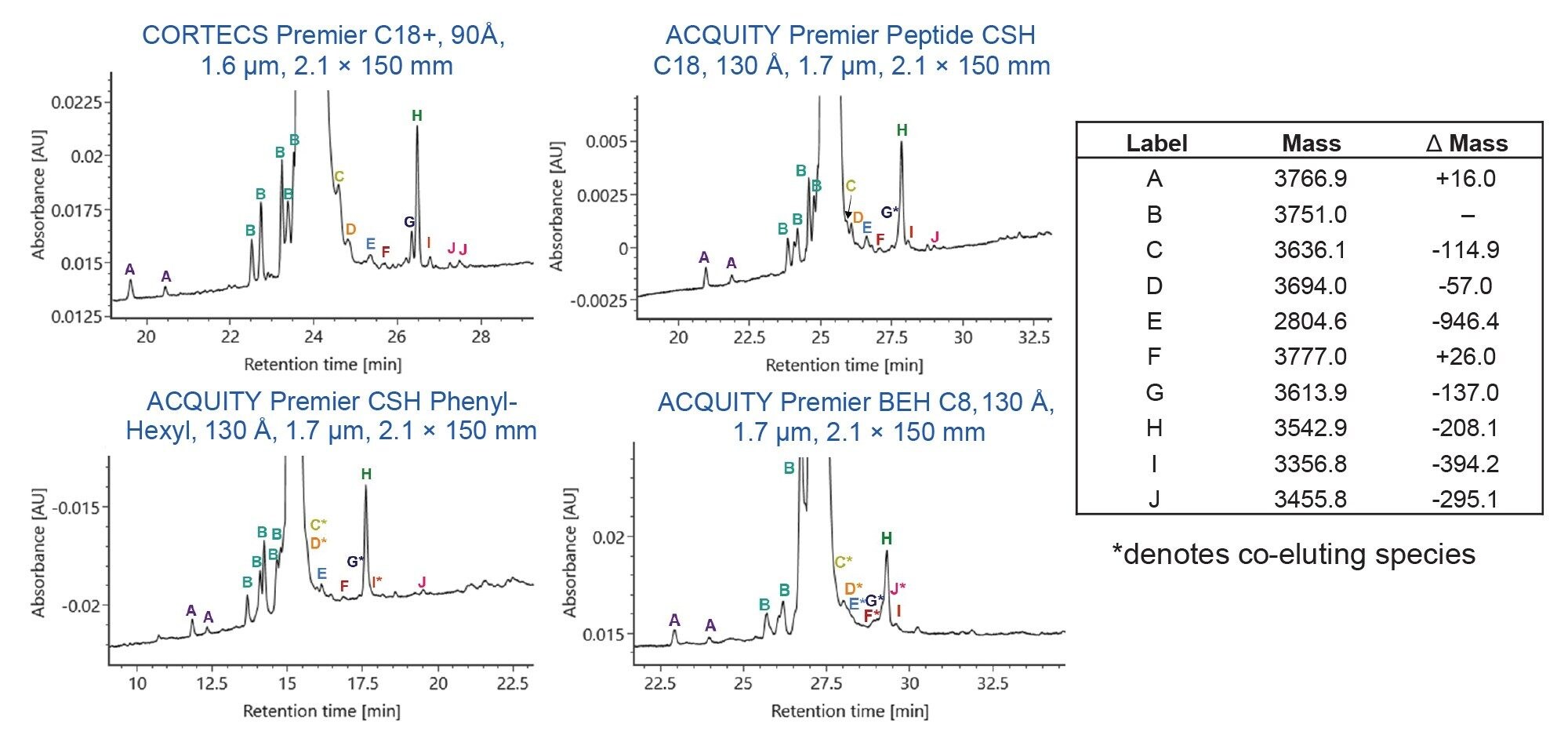
Liraglutide and Semaglutide Separation
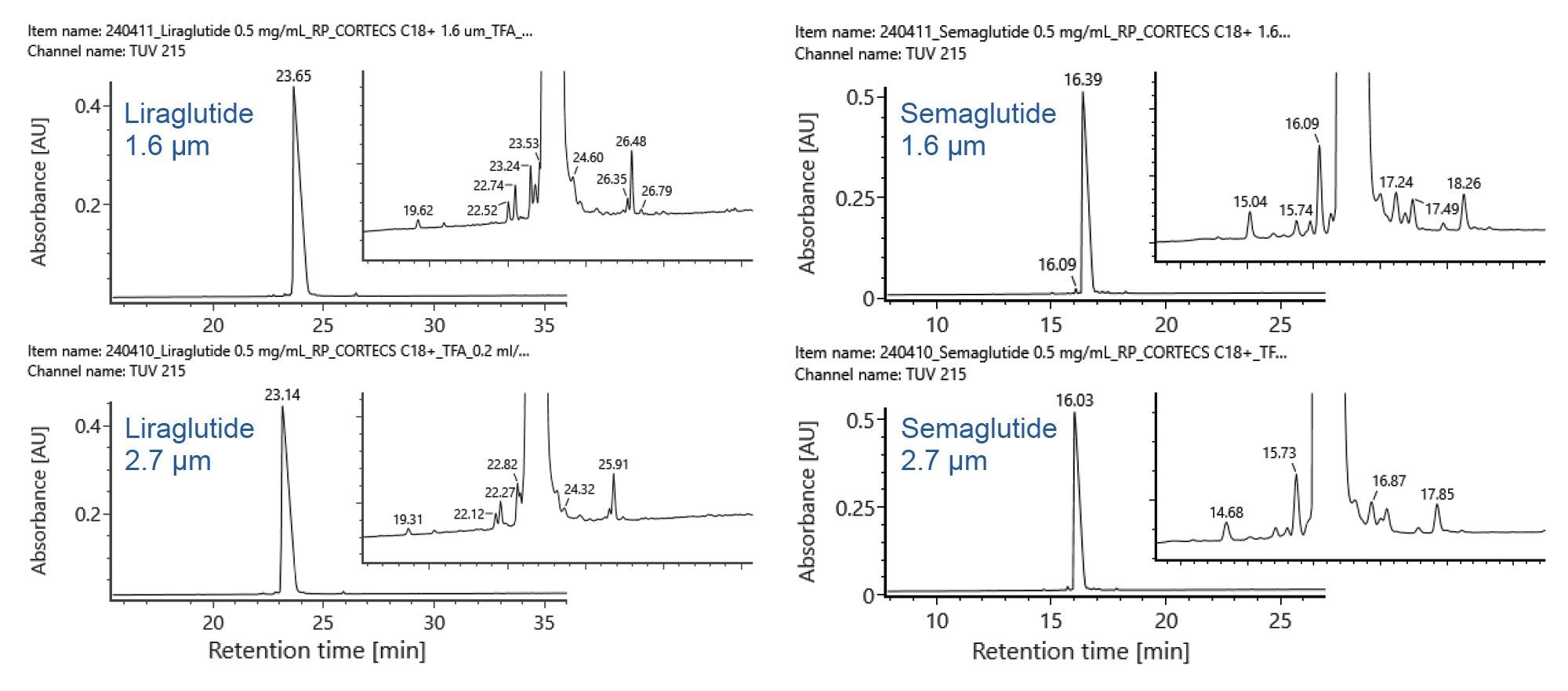
Figures 1 and 2 show the chromatographic results for liraglutide and semaglutide on the four selected columns. Peaks are labelled to highlight selectivity and resolution differences across the four columns and their mass shifts relative to the parent peak (Δ Mass) are shown in the tables on the right. Co-eluting species are marked with an asterisk. In Figure 1, peaks labelled “B” are isomers of liraglutide and in Figure 2, peaks labelled “D” are isomers of semaglutide.
Under these method conditions, the CORTECS Premier C18+ and the ACQUITY Premier Peptide CSH C18 columns isolate the most unique variants of liraglutide whereas the CORTECS Premier C18+ and the ACQUITY Premier CSH Phenyl-Hexyl columns isolate the most unique variants of semaglutide. The ACQUITY Premier Peptide CSH C18 Column yields better separation for liraglutide than semaglutide whereas the ACQUITY Premier CSH Phenyl-Hexyl Column yields better separation for semaglutide than liraglutide. While the ACQUITY Premier BEH C8 Column is not a top performer for either analyte, it effectively separates more variants of semaglutide than those of liraglutide.
These data demonstrate that minor differences in the chemical structure of fatty acid modified GLP-1 RAs impact column selection. Both liraglutide and semaglutide consist of modified human GLP-1 appended with a fatty acid chain. However, liraglutide contains a C-16 fatty acid linked through a glutamic acid spacer while semaglutide contains a C-18 fatty acid linked through a hydrophilic bis-aminodiethyoxyacetyl spacer.3 These differences in fatty acid chain length and spacer chemistry significantly impact the impurity separation on each of the studied columns. Column selection for RPLC-UV impurity analysis of fatty acid GLP-1 RAs therefore needs to be tailored to the specific analyte of interest. Regardless, the reported method and selected columns can be used as a starting point for analysts seeking a RPLC-UV method for impurity analysis of fatty acid modified GLP-1 RAs.
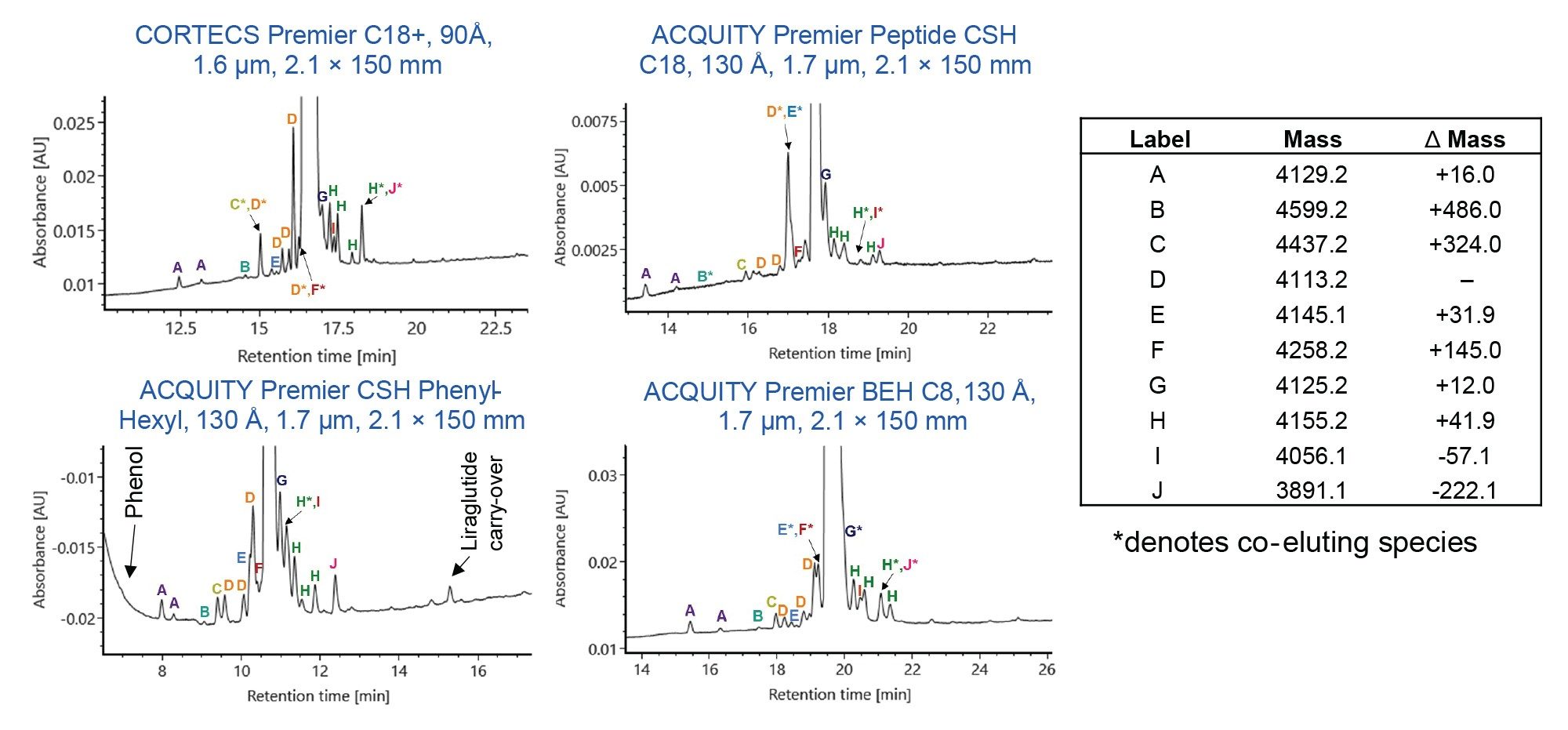
The CORTECS Premier C18+, 2.7 µm, 2.1 x 150 mm Column was also tested to evaluate the effect of particle size on liraglutide and semaglutide impurity separation. Figure 3 shows the chromatographic results for liraglutide and semaglutide on the CORTECS Premier C18+ 1.6 µm and 2.7 µm columns. Using the same gradient conditions and column size, a predicted loss of resolution and impurity detail is observed. However, a reasonable separation is still achieved using the 2.7 µm particle, demonstrating potential scalability of the method from UPLC to UHPLC/HPLC. A longer 4.6 x 300 mm CORTECS Premier C18+ 2.7 µm Column could be employed if additional resolution is required.
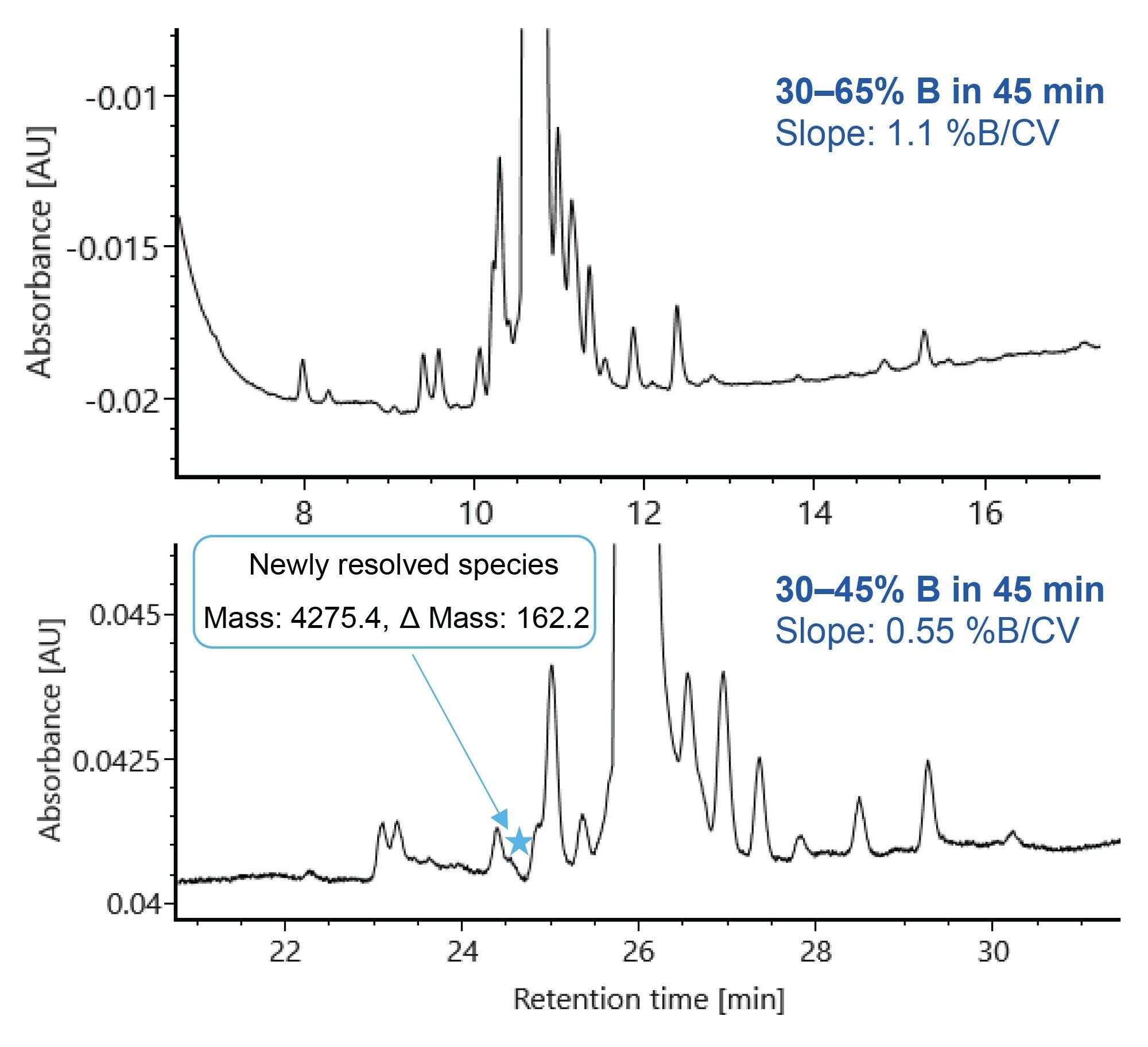
Gradient Optimization for Semaglutide Separation
The column comparison data shown above used a single gradient for all columns and analytes to simplify column-to-column comparison. The gradient for semaglutide separation on the ACQUITY Premier CSH Phenyl-Hexyl Column was optimized to improve separation of impurities and eliminate interference with phenol. The RPLC-UV chromatograms for semaglutide on the ACQUITY Premier CSH Phenyl-Hexyl Column using the GLP-1 RA screening gradient and an optimized gradient are shown in Figure 4. The optimized gradient improves the separation of semaglutide variants and reveals additional species that were not observed with the screening gradient. As highlighted in Figure 4, a new species at 24.6 minutes is seen with the optimized gradient.
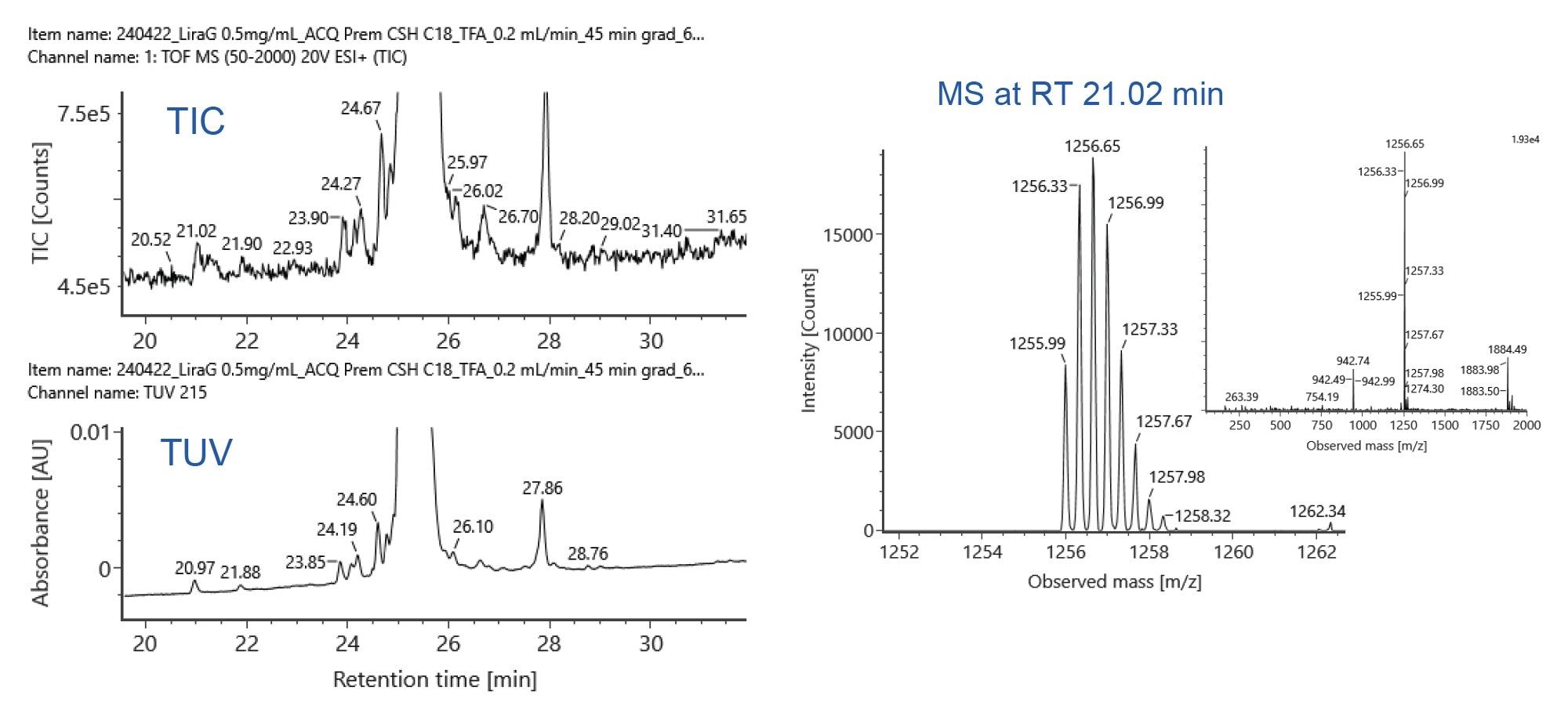
MS Compatibility
TFA was used as the mobile phase additive in these studies for its optical baseline noise and peak capacity advantages. TFA is typically avoided for MS detection because of its propensity to suppress ionization and contaminate MS systems.4 Figure 5 shows the TIC and UV chromatograms of liraglutide on the ACQUITY Premier Peptide CSH C18 Column (left) and the mass spectrum of the TIC peak at 21.02 min (right). The mass spectrum shown in Figure 5 correlates to a species with a mass of 3766.9 Da (mass shift relative to liraglutide: 16.0 Da, oxidized liraglutide). This data demonstrates that despite ion suppression from TFA, high-quality MS data can be obtained and peaks as low as 0.1% abundance relative to the main peak can be identified.
TIC and UV chromatograms of liraglutide on the ACQUITY Premier Peptide CSH C18 Column using 0.1% TFA or 0.1% formic acid mobile phase additives are shown in Figure 6. MS signal significantly increases when formic acid is used in place of TFA and differences in selectivity and resolution are observed between the two mobile phase additives. Thus, if improved MS signal is required or MS contamination with TFA is a concern, formic acid can be used as an alternative mobile phase additive. However, use of formic acid in place of TFA may result in lower peak capacity, noisier UV baselines, and selectivity differences.

Conclusion
The increased demand and use of GLP-1 RAs prompts a need for robust chromatographic methods to screen for impurities that may impact their safety and efficacy. Here, four column chemistries were screened for the RPLC-UV analysis of two fatty acid modified GLP-1 RAs, liraglutide and semaglutide. These studies show that no single column will provide optimal separation for all fatty acid modified GLP-1 RA constructs. Therefore, column screening should be considered when developing a RPLC-UV impurity analysis method for fatty acid modified GLP-1 RAs. The columns and chromatographic conditions presented here serve as additional direction for the development of RPLC-UV methods for fatty acid modified GLP-1 RAs. Moreover, we anticipate this methodology to be effective for the impurity analysis of non-GLP-1 RA lipopeptides.
References
- Watanabe, J.H., Kown, J., Nan, B., Reikes, A. Trends in Glucagon-like Peptide 1 Receptor Agonist use, 2014 to 2022. Journal of the American Pharmacists Association. 2024, 64, 133–138.
- Clements, B.R., Rainville, P. Development of Separation Methods for GLP-1 Synthetic Peptides Utilizing a Systematic Protocol and MaxPeak™ High Performance Surface Technology. Application note. 720008267. Waters Corporation. 2024.
- Knudsen, L. B., Lau, K. The Discovery and Development of Liraglutide and Semaglutide. Frontiers in Endocrinology. 2019, 10, 155.
- Koza, S. M. Chambers, E. E. Selecting a Reversed-Phase Column for the Peptide Mapping Analysis of a Biotherapeutic Protein.Application note. 720005924. Waters Corporation. 2017.
Featured Products
720008509, September 2024