Development of Separation Methods for GLP-1 Synthetic Peptides Utilizing a Systematic Protocol and MaxPeak™ High Performance Surface Technology
Abstract
GLP-1s are synthetic peptide drugs used to treat type-II diabetes and obesity. Recently, GLP-1s, such as semaglutide, have risen in demand. Currently, there is not a single method capable of separating and identifying a full panel of the GLP-1s currently being prescribed today. In this application, we developed a reproducible HPLC-UV/MS method that covers a variety of GLP-1s currently on the market. We also show the capability of this method to separate and identify related impurities. Finally, we demonstrated the benefits of using MaxPeak™ High Performance Surface (HPS) Technology, when compared to traditional stainless-steel systems, for synthetic peptide analysis.
Benefits
Introduction
Glucagon-Like Peptide-1 Agonists (GLP-1s) are a class of synthetic peptide medications that are prescribed for the treatment and management of obesity and type-II diabetes1. Recently, drugs such as semaglutide, have boomed in popularity as a weight management treatment after success in clinical trials.2 Given the prevalence of GLP-1s, it is important that the quality control for this class of pharmaceuticals is supported by versatile, sensitive, and reproducible chromatography methods. While there are chromatographic methods for some of the common GLP-1s, to our knowledge, there is not a single method to separate an updated panel of this class of synthetic peptides.3–6 Further, the U.S. Food and Drug Administration (FDA) recently released Product Specific Guidelines (PSG) for some of the synthetic peptides on the market.7 In this presentation, the FDA states the importance for impurity analysis of synthetic peptides.
Here we address these needs and developed a single HLPC-UV/MS method for the analysis of a variety of GLP-1s utilizing a systematic protocol for method development in combination of Waters™ MaxPeak HPS. This technology has previously been shown to mitigate undesirable metal and peptide interactions leading to improvements in chromatographic separation parameters such as chromatographic peak area count, tailing and retention time reproducibility.8,9
Experimental
Stock Standard Preparation
Dulaglutide, Exenatide, Glucagon, Lixisenatide, and Tirzepatide were purchased from Selleck Chem (Houston, TX). Liraglutide and Semaglutide (acetate) were purchased from Cayman Chemical (Ann Harbor, MI). Stock concentrations varied based on solubility and amount of material purchased (Table 1). Any salt factors were taken into consideration during preparation. Some sonication was used to dissolve standards into solution.
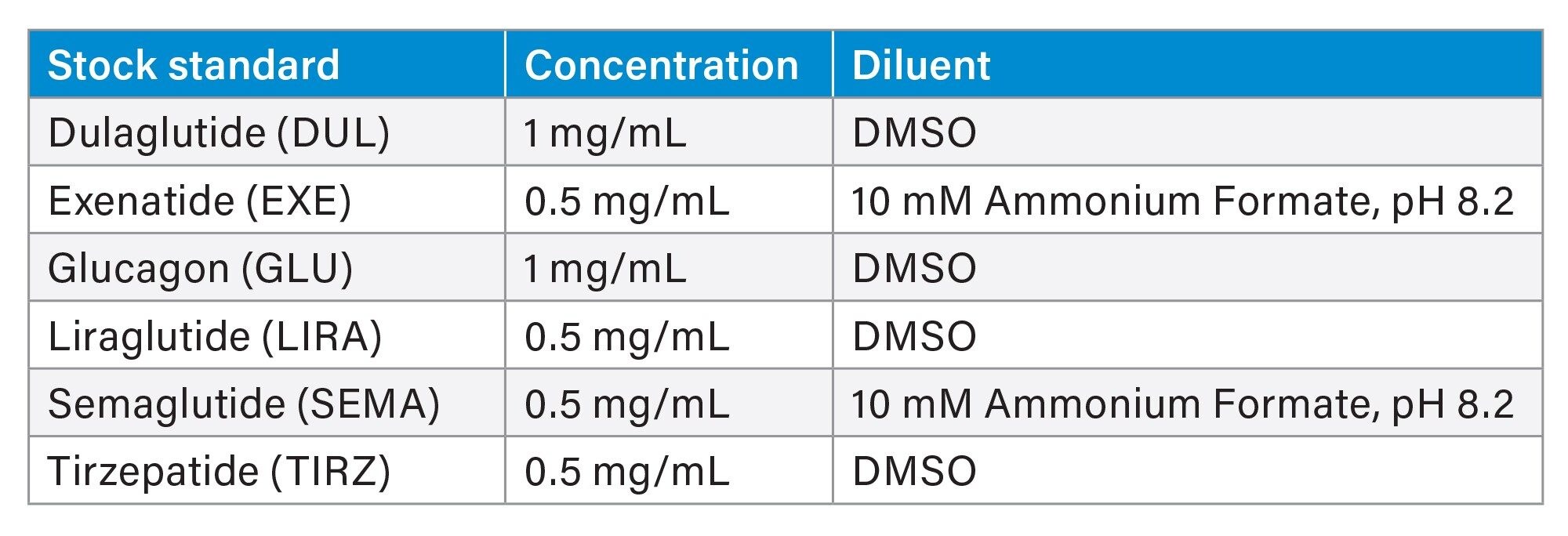
Stocks were stored at 2 °C–8 °C. Given the physical properties of DMSO, stocks solidified in cold storage. Thawed stocks were allowed to equilibrate to ambient room temperature prior to standard preparation.
Standard Preparation
Stocks were diluted using 0.1:0.5:99.4 Trifluoracetic Acid: Acetonitrile: Deionized Water (standard diluent). Stocks were combined and prepared so that all analytes were at a 100 µg/mL concentration (GLP-1 Drug Panel).
Further, a separate standard containing only Glucagon was made to demonstrate the use of focused gradients and how it can be used to optimize the separation of impurities. This standard was created by diluting the individual glucagon stocks to a 750 ug/mL concentration using the standard diluent.
LC Conditions
Gradient Table for GLP-1 Panel Screen Gradient
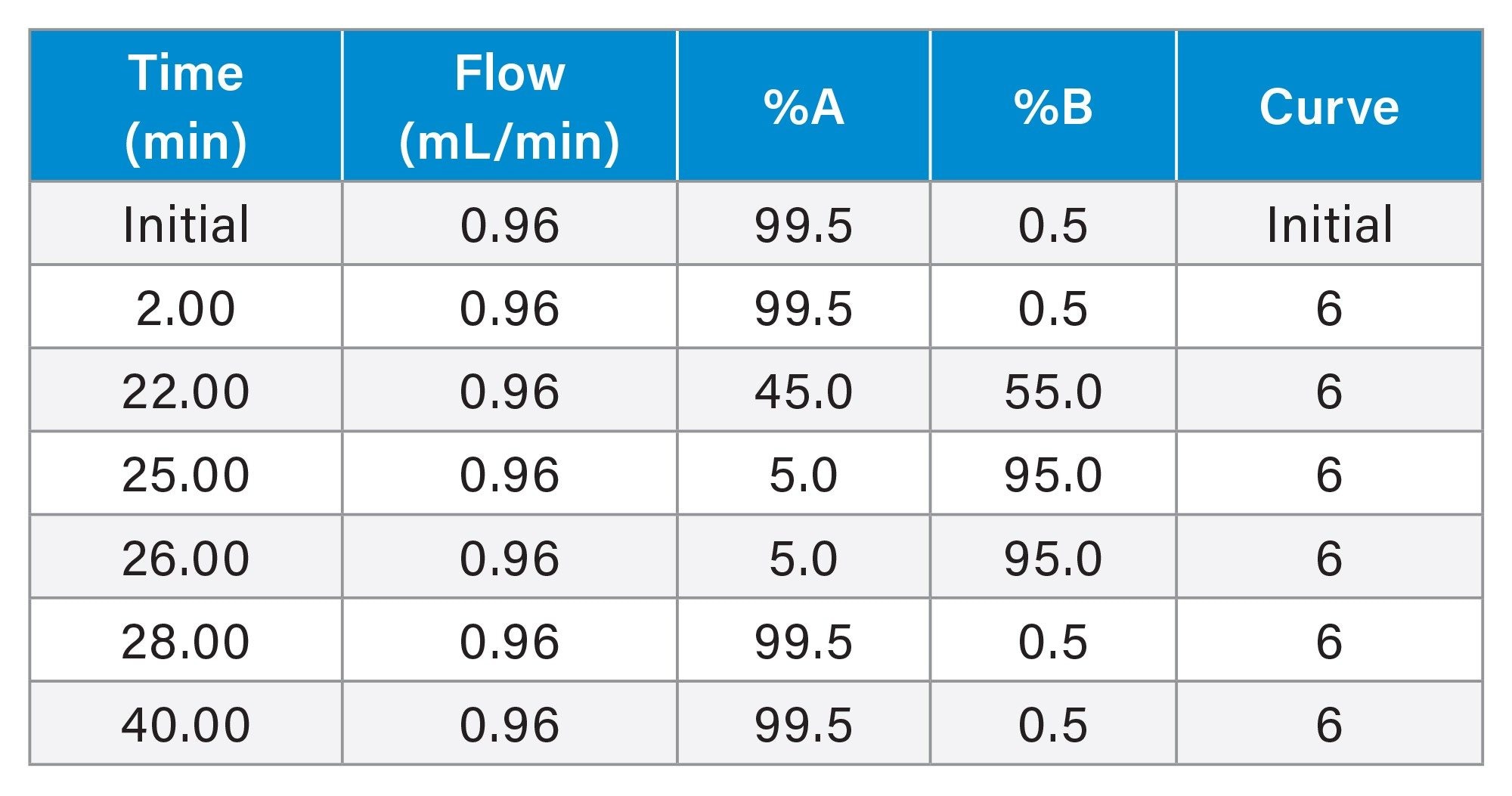
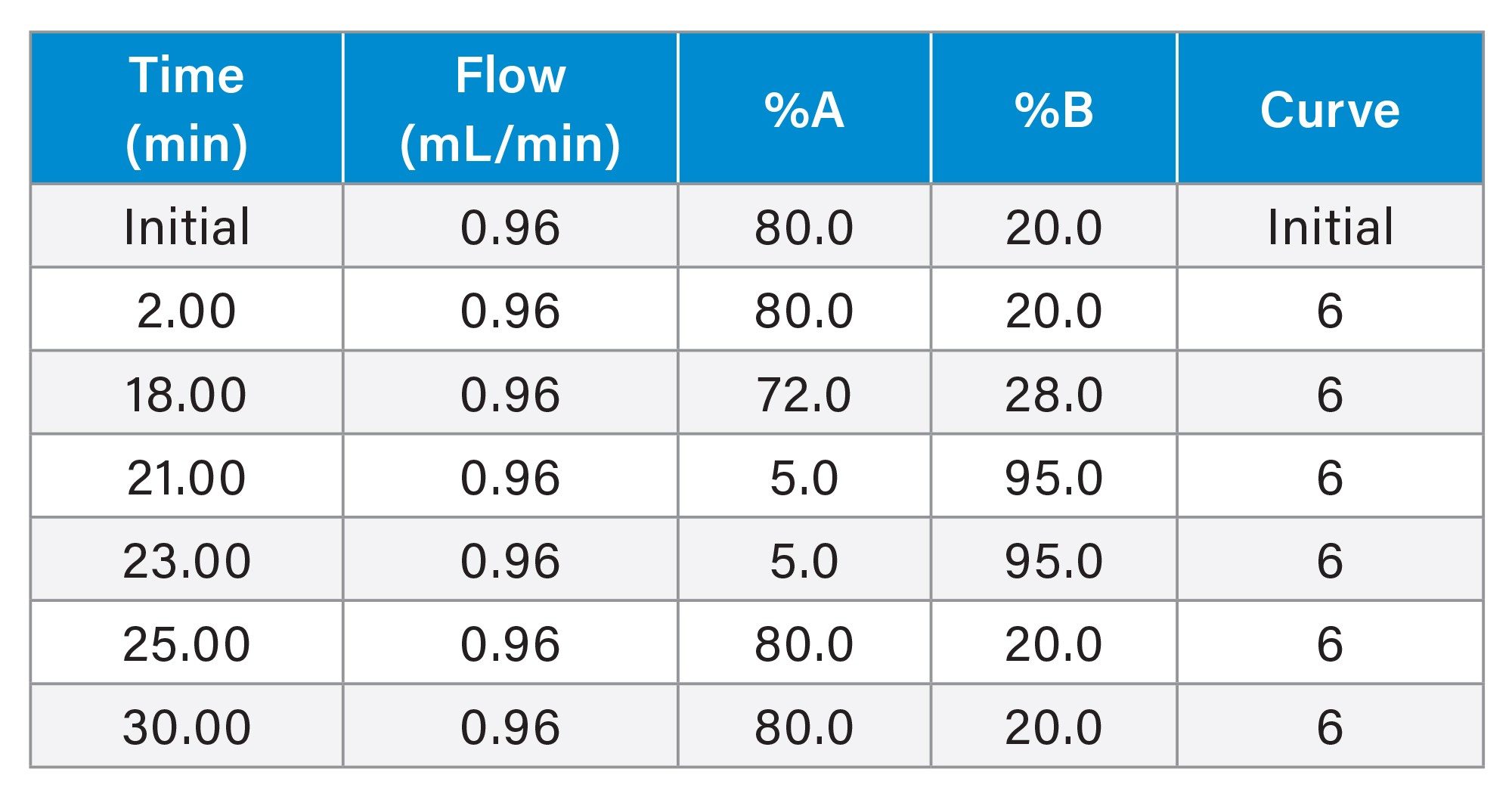
Gradient Table for Glucagon Focused Gradient
Data Management
|
Instrument control: |
Empower™ 3.7.0 |
|
Data processing: |
Empower 3.7.0 |
Results and Discussion
Method of Separation Using a Screening Gradient
The systematic protocol approach for method development suggests screening a variety of columns and reversed phase mobile phase combinations to determine the most appropriate conditions to optimize chromatographic separations.
Four different columns and mobile phase eluent combinations were investigated as defined in the MaxPeak Premier Reversed-phase Column Screening Kit (Table 2).
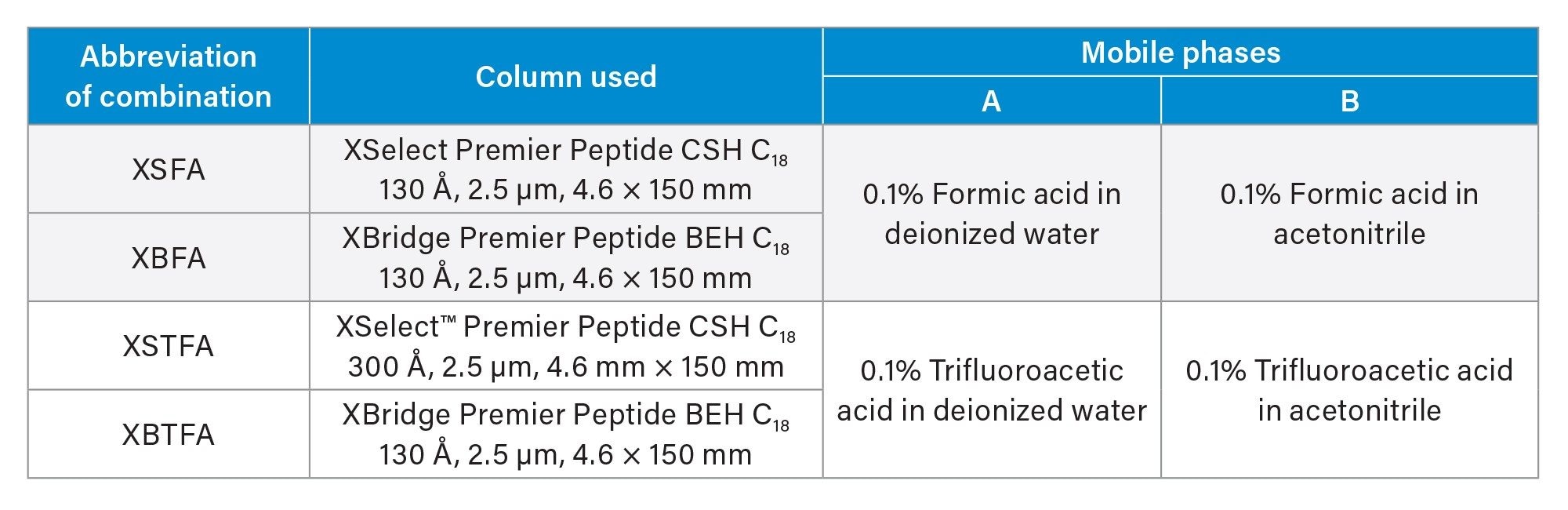
A representative chromatogram of each set of conditions clearly demonstrated the difference in retentivity when using different columns and mobile phase combination in Figure 1 for the GLP-1 panel utilized in this work.
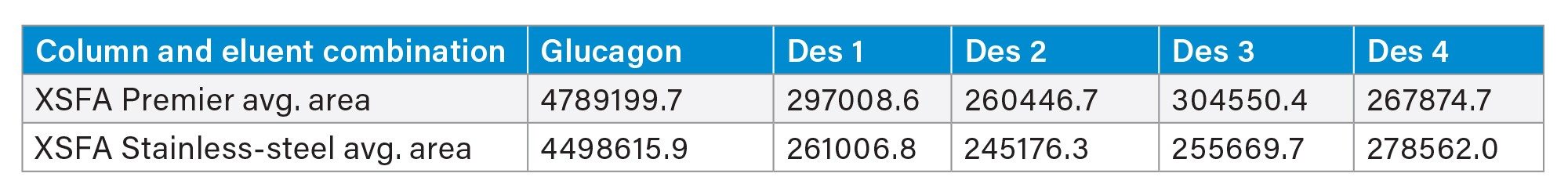
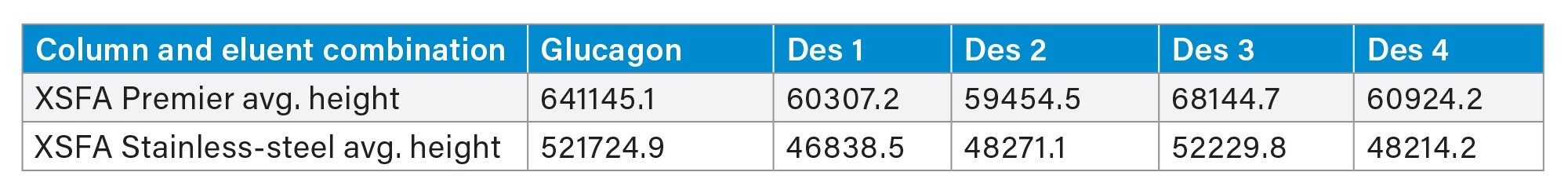
Based on our screening experiments we selected the XSelect Premier Peptide CSH C18 Column with the formic acid mobile phases for our method due to the overall speed and resolution these combinations of method conditions produced. We further chose these conditions based on the maximized chromatographic peak height performance of glucagon (Table 3).

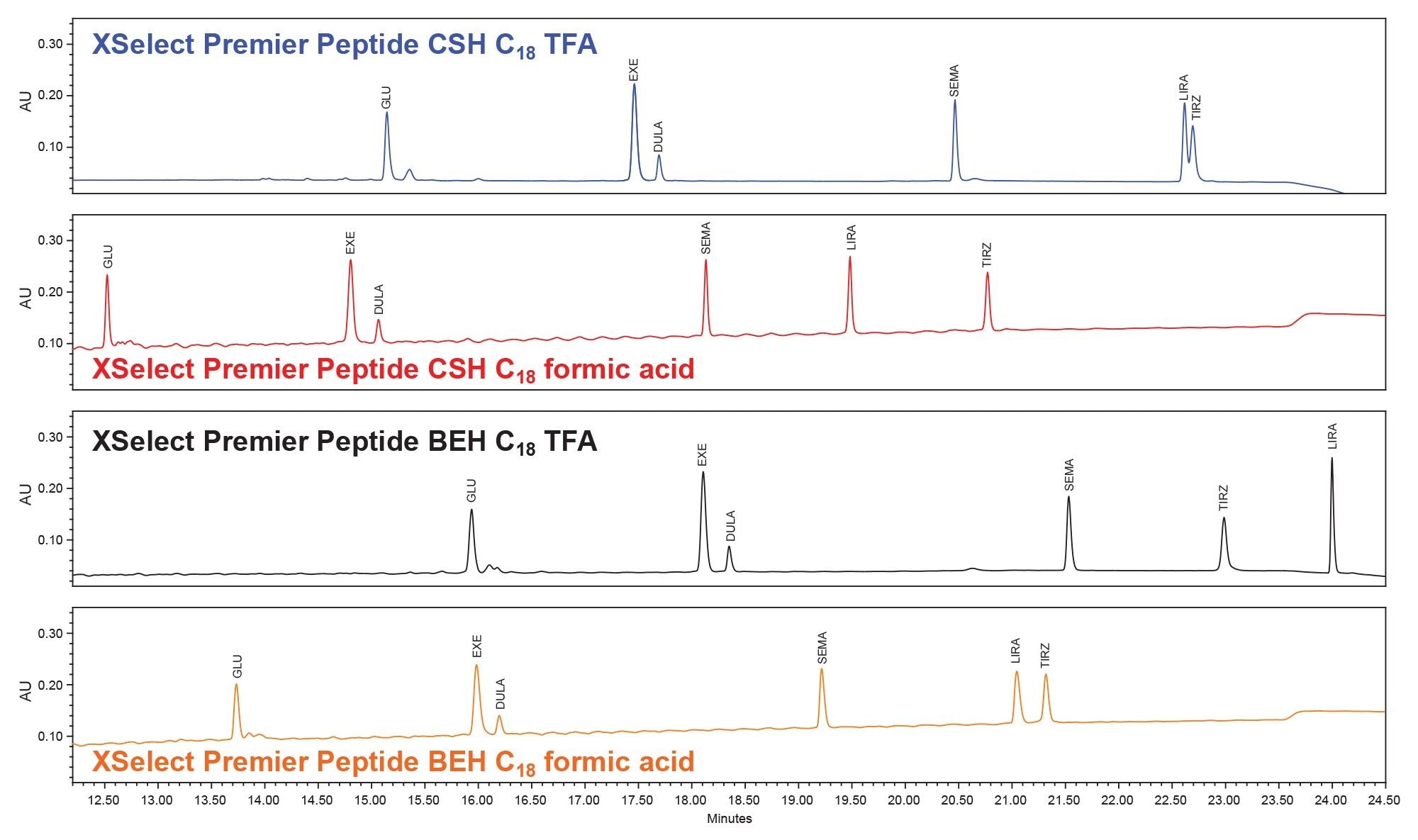
Further, the XSelect Premier Peptide CSH C18 Column provided better separation of the glucagon desamido impurities when compared to the other column and mobile phase combinations as shown in Figure 2. Glucagon has four common desamido impurities, which are a result of deamination of the peptide’s amino acid residues.4,11
Focused Gradient to Separate Glucagon Impuirites
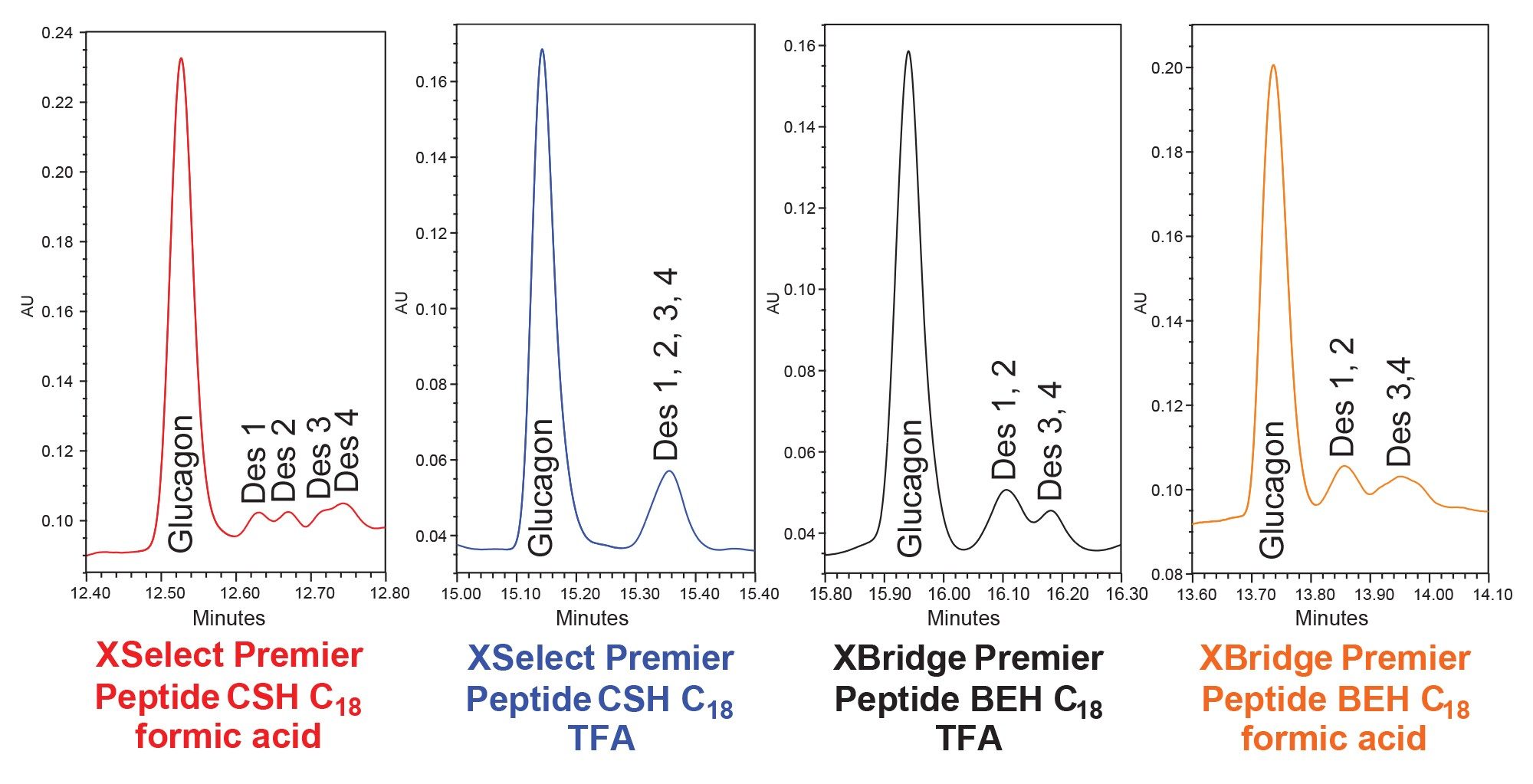
In Figure 2 we can see that the glucagon desamido impurities are poorly resolved using the initial screening gradient. To improve the separation of these impurities, we used a focus gradient in conjunction with the XSelect Premier Peptide CSH C18 Column and formic acid mobile phases as it is described in the MaxPeak Premier Peptide Reversed-phase Column screening Kit. The gradient details are presented previously in the gradient table within the method conditions section. In Figure 3 we show the results of the optimized focused gradient for glucagon and associated desamido impurities.
The peak purity for glucagon and the desamido impurities was confirmed with UV and mass spectra data obtained from the PDA detector and ACQUITY QDa Mass Detector (Figure 4a through 4c).
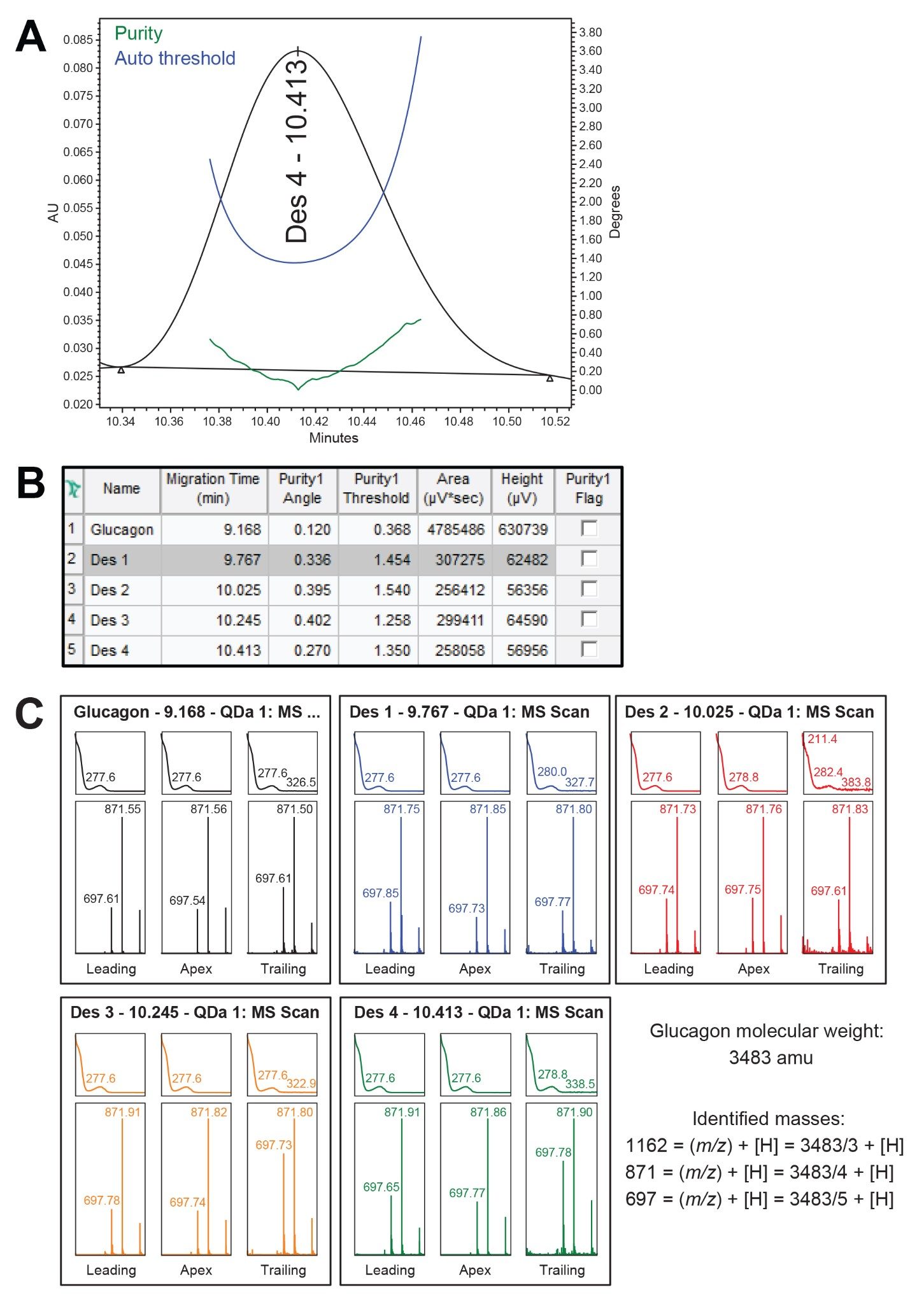
Figure 4a. An example of the UV PDA peak purity of the Des 4 chromatographic peak using the peak purity tools within Empower. Here the purity threshold is below the auto threshold indicating the peak is pure.
Figure 4b. Tabulated data associated with peak purity for glucagon and its desamidos using the peak purity tools in Empower.
Figure 4c. Mass spectral data results from the ACQUITY QDa Mass Detector for a representative injection of the glucagon individual standard. Here we show, the leading, apex, and trailing aspects of the MS scan of glucagon and each of its desamido peaks. Distinct ions are shown in each of the plots. The ions are associated with charged states of the glucagon peptide. Based on the mass spectra, and UV spectra, the data suggests each of the desamidos are related to glucagon.
Comparison of Premier Technology to Tradition Stainless Steel
During our investigation of the different column and mobile phase combinations, we examined the effects that Premier Columns and Systems featuring MaxPeak HPS had on the GLP-1 Drug Panel. The results of some analytes in the panel were compared to results obtained using a traditional stainless-steel columns and systems.
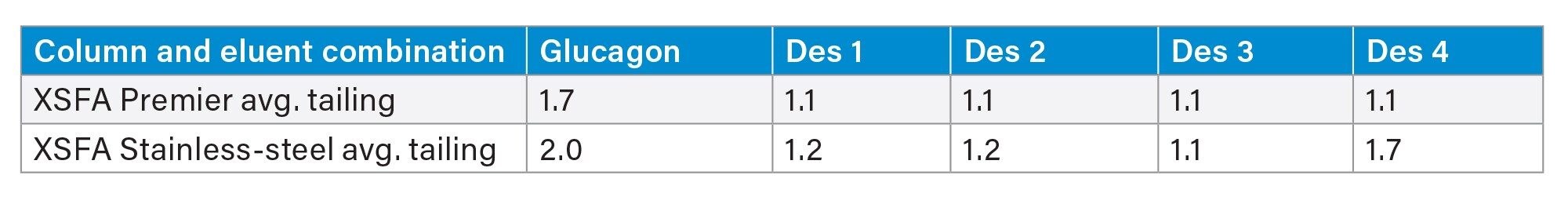
When using a focused gradient on XSelect Peptide CSH C18 Columns and formic acid mobile phases to separate glucagon and its impurities, we found that MaxPeak HPS increased the area by up to 19%, increased the height by up to 30%, and decreased the tailing by up to 36% (Figure 5, Tables 4 through 6).

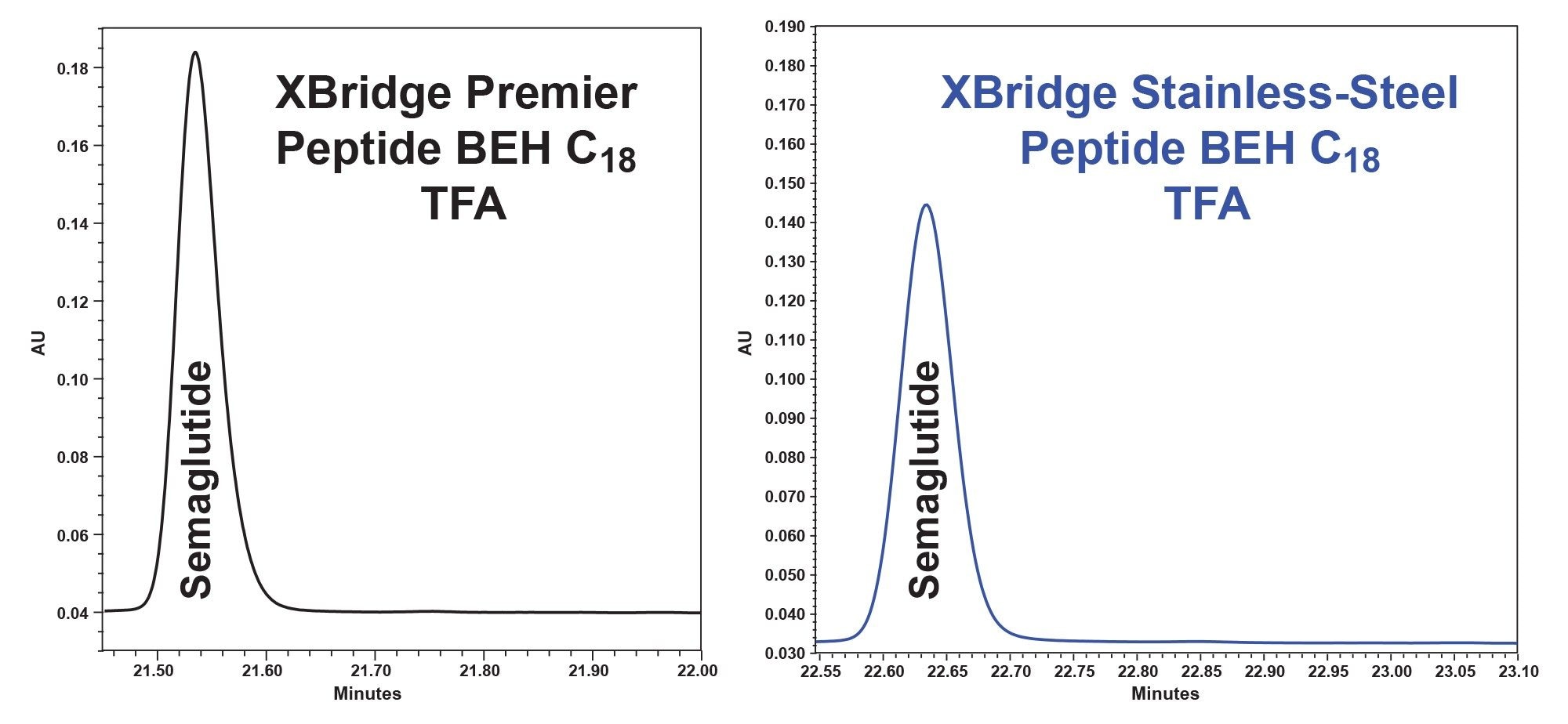
When using a screening gradient with the XBridge Peptide BEH C18 Column using trifluoro-acetic acid eluents to separate semaglutide in the GLP-1 Drug Panel mix, we found that MaxPeak HPS increased the area by 20% and increased the height by up to 30% (Figure 6, tables 7 through 8).

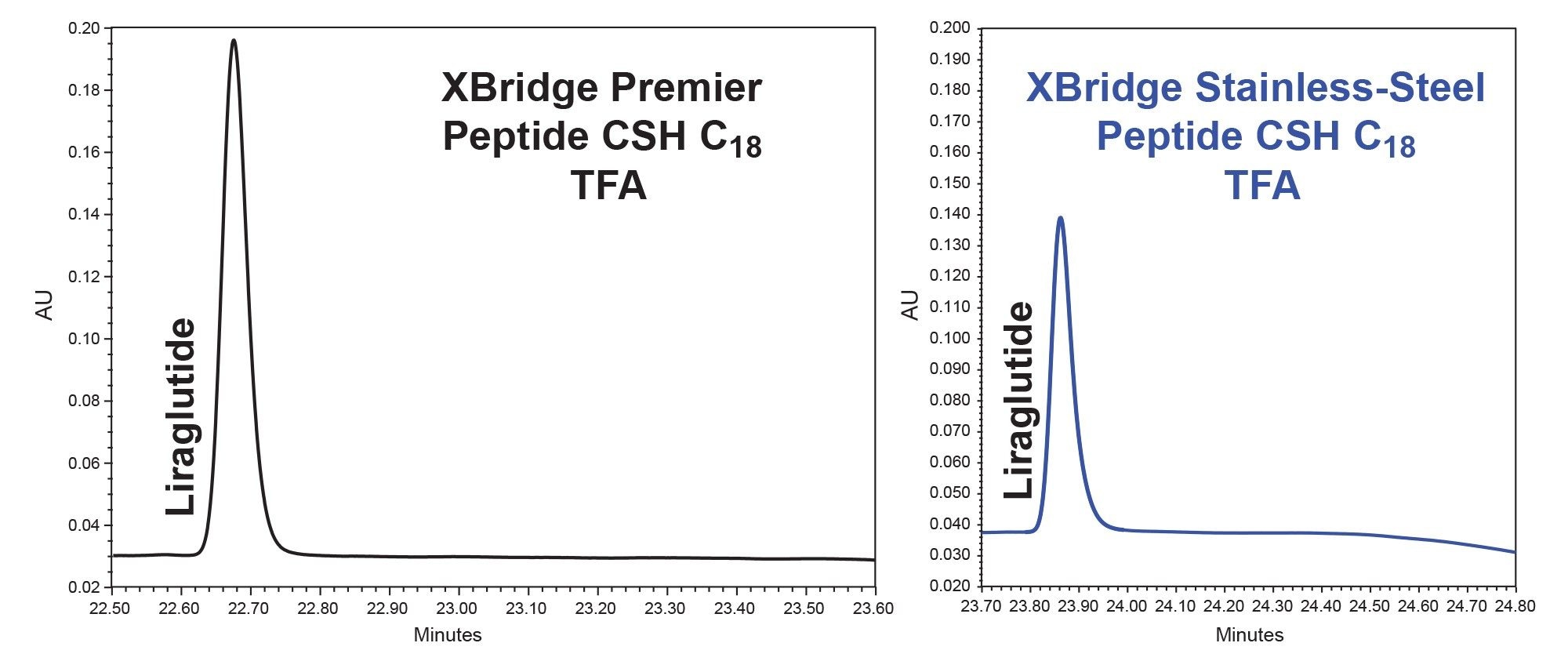
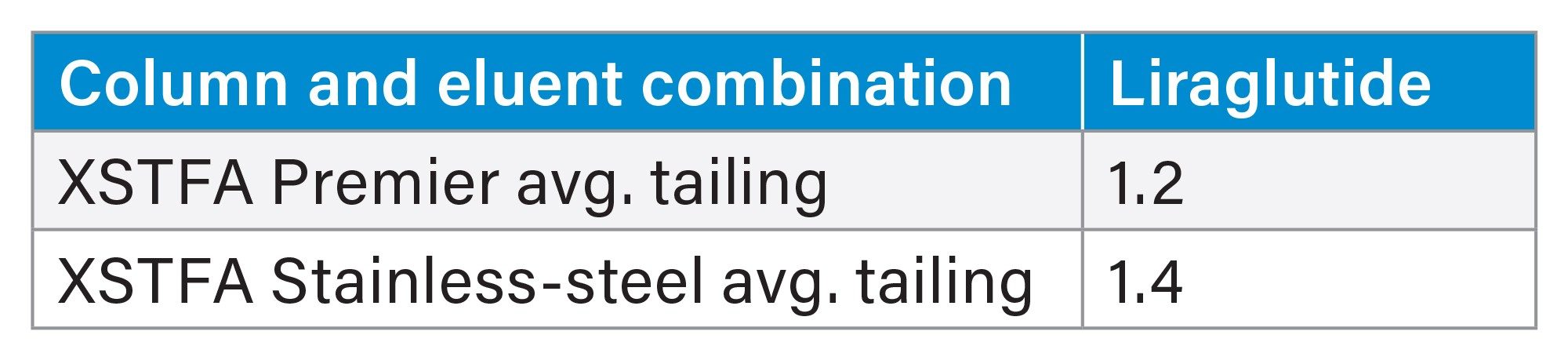
Finally, when using a screening gradient with the XSelect Peptide CSH C18 Column using trifluoro-acetic acid eluent to separate liraglutide in the GLP-1 Drug Panel mix, we found that MaxPeak HPS increased the peak area by 28%, increased the peak height by 51%, and decreased peak tailing by 13% (Figure 7, tables 9 through 11).

Conclusion
In this application note, we demonstrated the utilization of the systematic protocol outlined in the MaxPeak Premier Peptide Reversed-phase Column Screening Kit. This method development approach produced quality separations of glucagon like peptides (GLP- 1s), glucagon and associated desamido impurities. Glucagon and desamido impurities were base line separated and detected by both UV and MS detection. The purity of the chromatographic peaks was determined through the Empower Peak purity processing tool, providing information on desamido relatedness to glucagon. The use of MaxPeak HPS Technology provided increases in chromatographic peak area of up to 28%, peak height of up to 51%, and reduction in peak tailing of 13% for a variety of GLP-1s tested.
References
- Holz G, Chepurny O. Glucagon-Like Peptide-1 Synthetic Analogs: New Therapeutic Agents for Use in the Treatment of Diabetes Mellitus. Current Medicinal Chemistry. 2003 Nov 1;10(22):2471–83.
- Magda Sara Wojtara, Mazumder A, Syeda Y, Nikodem Mozgała. Glucagon-Like Peptide-1 Receptor Agonists for Chronic Weight Management. Advances in medicine. 2023 Sep 20;2023(Article ID 9946924):1–7.
- United States Pharmacopeia. Exenatide Injection USP Monograph. online.uspnf.com. 2019.
- United States Pharmacopeia. Glucagon USP Monograph. online.uspnf.com. 2022.
- Kumar Kuna A, Ganapathi S, Radha G. A Novel RP- HPLC Method Development and Forced Degradation Studies for Semaglutide in Active Pharmaceutical Ingredients and Pharmaceutical Dosage Form. International Journal of Research in Pharmaceutical Sciences. 2019 Apr 15;10(2):865–73.
- Pedaprolu JN, Bonthu M, Vatchavai B, Kamatham S, Kolli S, Kapuganti AN. A New Stability-Indicating and Validated RP-HPLC Method for the Estimation of Liraglutide in Bulk and Pharmaceutical Dosage Forms. Eurasian Journal of Analytical Chemistry. 2016 Dec 8;12(2):31–44.
- Pang E. Assessing Immunogenicity Risk of Peptides: the Synthetic Peptide Guidance and PSGs SBIA 2022: Advancing Generic Drug Development: Translating Science to Approval Day (1), Session 1A: (Peptide Immunogenicity Risk and Impurity Assessment Considerations) [Internet]. U.S. Food and Drug Administration; 2020 [cited 2023 Nov 6]. Available from: https://www.fda.gov/media/166571/download.
- Birdsall R, Kellet J, Ippoliti S, Qing Yu Y. Increasing Recovery and Chromatographic Performance of “Acidic” Peptides Using Waters ACQUITY Premier Solution [Internet]. www.waters.com. Waters Corporation; 2021. Available from: 720007173. March 2021.
- Bigos P, Birdsall R, Walter T. MaxPeak Premier Solutions: Improving Consumer Safety Through Innovative Science [Internet]. www.waters.com. Waters Corporation; 2021. Available from: 720008054.
- MaxPeak Premier Peptide Reversed-Phase Column and Method Screening Kit: Practical Steps in Developing Robust Peptide Separations Available from 720008131.
- Bao Z, Cheng YC, Luo MZ, Zhang JY. Comparison of the Purity and Impurity of Glucagon-for-Injection Products under Various Stability Conditions. Scientia Pharmaceutica [Internet]. 2022 Jun 1 [cited 2023 Nov 10];90(2):32. Available from: https://www.mdpi.com/2218-0532/90/2/32.
720008267, March 2024