High Utilization and Robustness of the Alliance™ iS HPLC System Using the Analysis of a USP Impurity Method
Abstract
In many regulated laboratories, there is a need for reliable, robust High Performance Liquid Chromatography (HPLC) systems to accommodate the demand for high instrument utilization and product timelines. With these types of restraints, regulated laboratories need an HPLC system capable of operating for extended periods of time with minimal downtime. The high utilization of the HPLC system must also produce high-quality, reliable results. The Alliance iS HPLC System is a streamlined system with features that help to reduce human error in system operation, thereby decreasing the need to rerun analyses. One example of a feature on the Alliance iS HPLC System is the modernized interface that enhances the ease of use by HPLC users to maximize the system’s uptime and efficiency. In this application note, the Alliance iS HPLC System ran for a time period of forty days with consistent, accurate results of the USP Fluconazole Organic Impurities method.1 Over the course of the study, a system suitability solution and a Fluconazole sample containing impurities were monitored each day to assess system suitability.
Benefits
- The Alliance iS HPLC System has the capability to efficiently run for extended periods of time
- The Alliance iS HPLC System provides accurate results when being utilized for analyses over a long period of time
- The Alliance iS HPLC System provides method robustness and consistency when analyzing a USP impurities method
Introduction
In many regulated laboratories, there is a need for reliable, robust instrumentation to meet ever increasing demands for instrument utilization and product timelines. With these types of constraints, regulated laboratories need HPLC systems capable of operating for extended periods of time with minimal downtime or high utilization (>75% of the time). This high utilization must also produce high quality, reliable results. One approach to address the challenge of efficiency and robustness is to implement a modernized technology with features that help to reduce human error in system operation, thereby decreasing the need to rerun analyses. The Alliance iS HPLC System is an HPLC System that was designed to enhance the ease of use for the HPLC user to maximize the system’s uptime and efficiency.2 By implementing a modernized interface and system pre-run checks, the Alliance iS HPLC System can help to improve productivity with enhanced robustness and uptime of the HPLC system.3 In this application note we will demonstrate how the Alliance iS HPLC System operated over forty days with consistent, accurate results of the USP Fluconazole Organic Impurities method. Over the course of the study, a system suitability solution and a Fluconazole sample containing impurities were monitored each day to assess system suitability.
Experimental
Sample Description
The Fluconazole Standard Solution was prepared per the USP monograph, with mobile phase (80:20 water: Acetonitrile) to give a final concentration of 10 µg/mL for each compound. Included in the Standard Solution were Related Compound A (Sigma-Aldrich, p/n: PHR1651-30 MG), Related Compound B (Sigma-Aldrich, p/n: PHR1862-20 MG), Related Compound C (Sigma-Aldrich, p/n: PHR1826-20 MG), and Fluconazole (Sigma-Aldrich, p/n: PHR1160-1 G). The System Suitability Solution is the same as the Standard Solution, therefore the same solution for the Standard Solution was also used for the System Suitability Solution. The Sample Solution, which used an expired reference standard (Sigma, p/n: F8929-100 mg) was weighed out and prepared in mobile phase with a final concentration of 3 mg/mL.
Method Conditions
LC Conditions
|
LC system: |
Alliance iS HPLC System with TUV Detector |
|
Detection: |
UV detection at 260 nm |
|
Vials: |
LCGC Certified Clear Glass 12 x 32 mm Screw Neck Vial, Max Recovery, with Cap, and Preslit PTFE/Silicone Septum (p/n: 186000327C) |
|
Column(s): |
XSelect™ HSS T3, 3.5 µm, 4.6 mm x 150 mm (p/n: 186004786) |
|
Column temperature: |
40.0 °C |
|
Sample temperature: |
10.0 °C |
|
Injection volume: |
20.0 µL |
|
Flow rate: |
0.5 mL/min |
|
Mobile phase A: |
Water |
|
Mobile phase B: |
Acetonitrile |
|
Isocratic: |
80:20 Mobile Phase A: Mobile Phase B |
|
Run time: |
20 minutes |
Data Management
|
Chromatography software: |
Empower™ 3.8.0.1 |
Results and Discussion
Quality Control (QC) laboratories face strict timelines to release products. The Alliance iS HPLC System is a modern technology that will assist in meeting the strict requirements to release products on time. The Alliance iS HPLC System was specifically designed to address the challenges regulated QC laboratories encounter.4 In this application one Alliance iS HPLC System was operated for forty days using the isocratic USP Fluconazole Organic Impurities method. Over the time period, the system suitability was analyzed every 24 hours to ensure that the system was operating properly and in a controlled state.
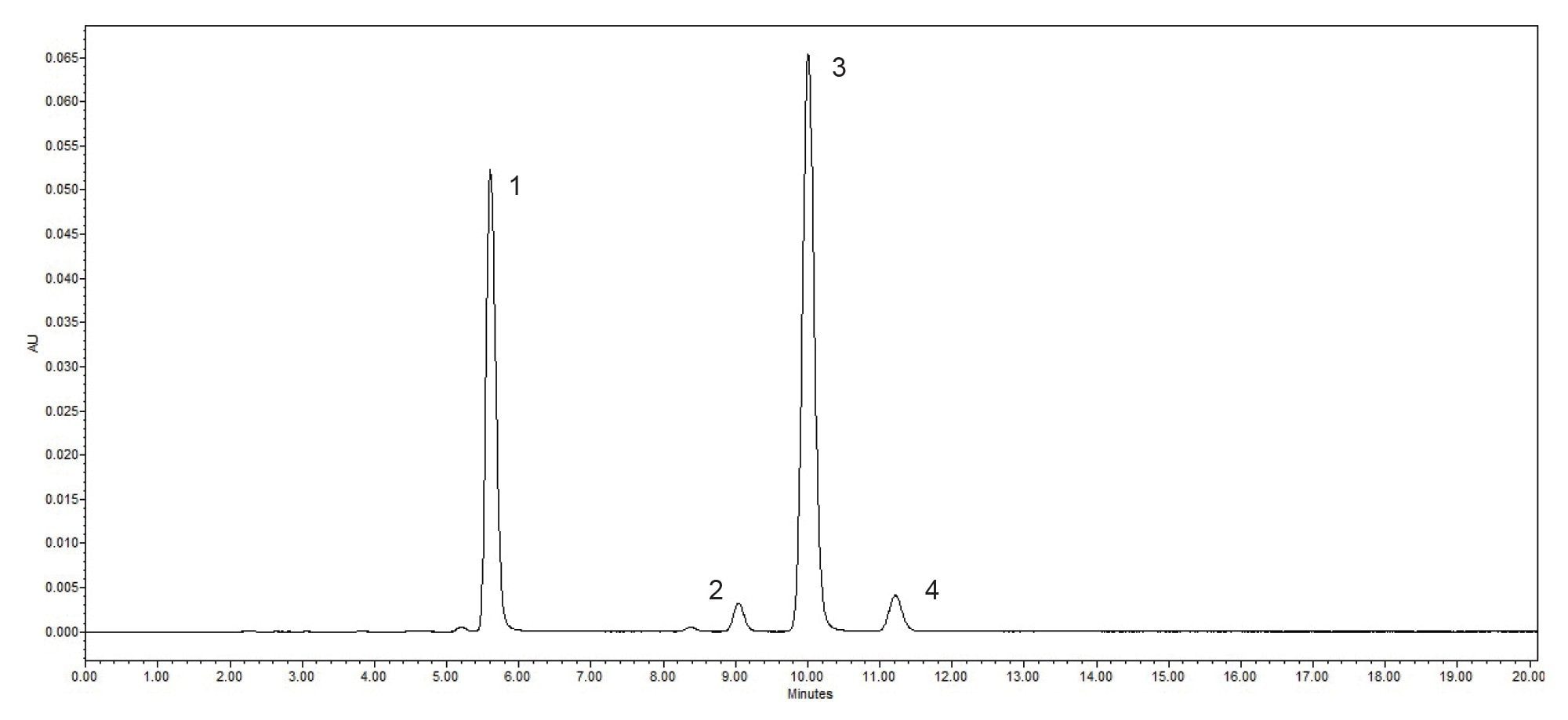
System suitability requirements found in the USP monograph are an assessment of the HPLC system to ensure that the system is performing correctly. For the USP Fluconazole Organic Impurities method, the system suitability states the resolution between Related Compound B and Related Compound C should be no less than (NLT) 1.5 in the system suitability solution. Also, the Area and Retention %RSD should be no more than (NMT) 5.0% for all compounds in the system suitability solution. Contained within the system suitability solution are the following four compounds, Related Compound A, Related Compound B, Related Compound C, and Fluconazole (Figure 1). The USP Fluconazole Organic Impurities Method was tested on an Alliance iS HPLC System for a period of 40 days. Data from all forty days of the system suitability solutions, Table 1, shows the Alliance iS HPLC System consistently, and reliably met the system suitability requirements for resolution, %RSD Area, and %RSD Retention Time easily for the USP Fluconazole Organic Impurities Method.
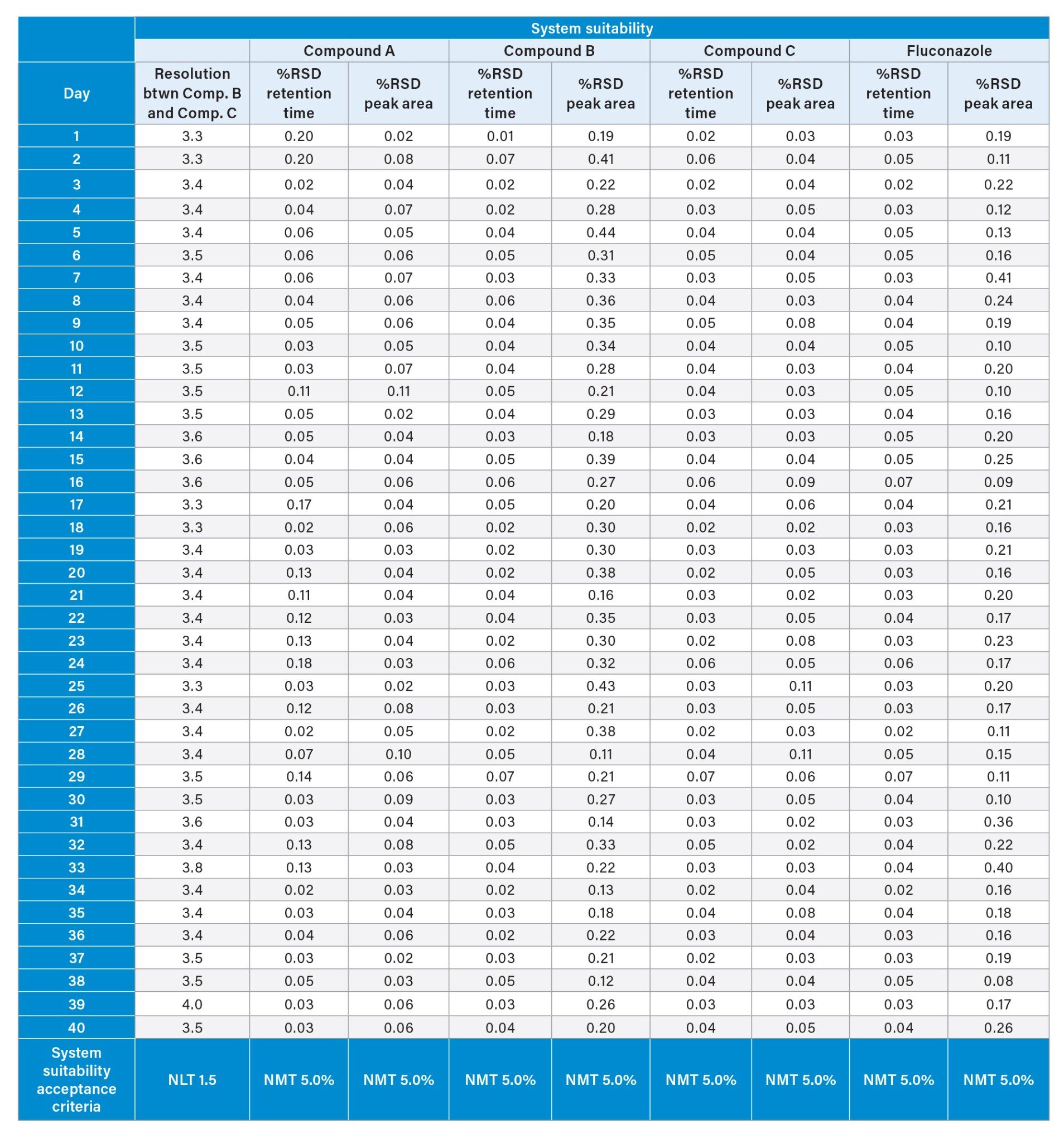
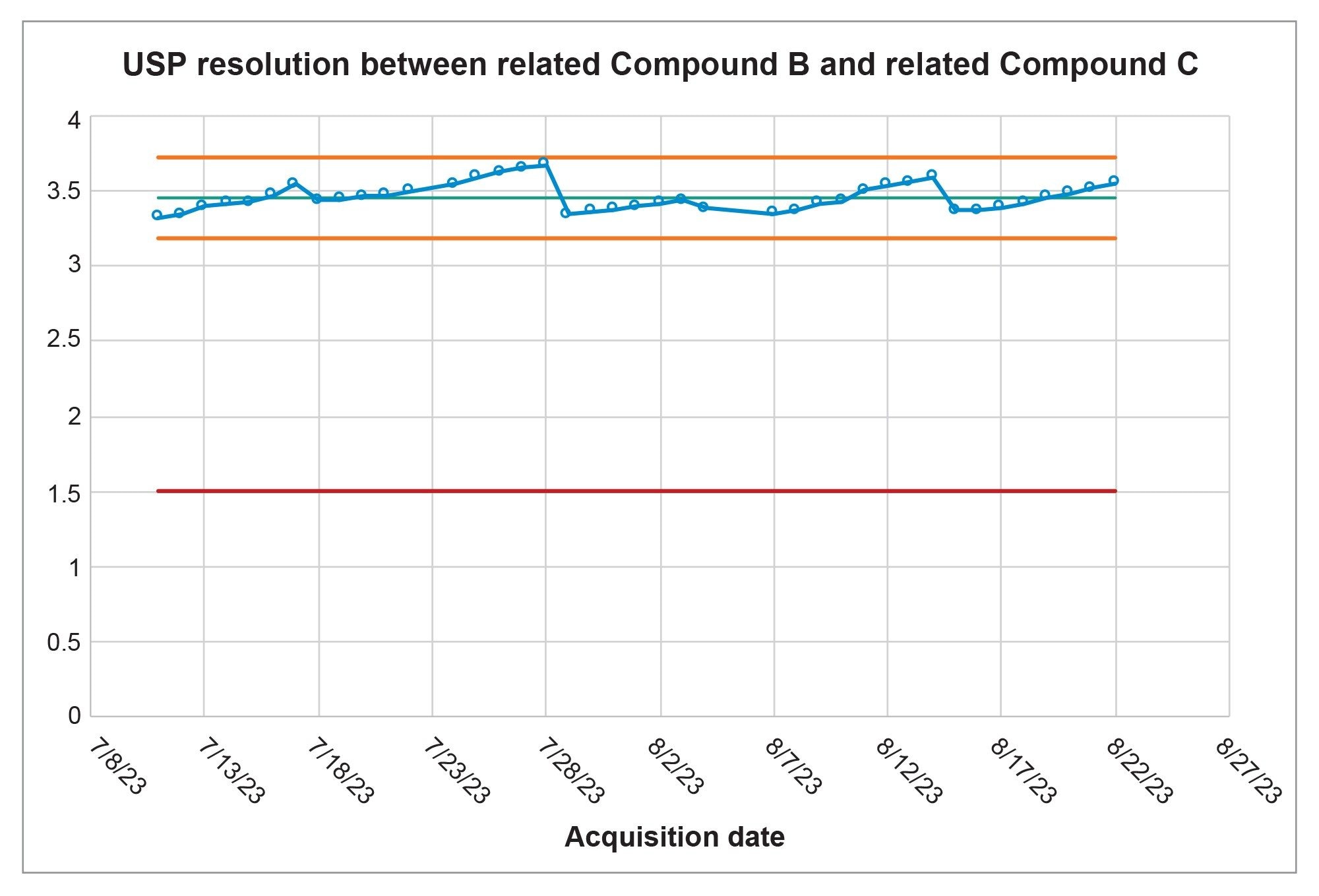
While meeting system suitability is required, the results were also monitored to assess the long-term performance and ensure that the Alliance iS HPLC System was in a state of control over the forty-day analysis. All of the data collected from the system suitability solutions were analyzed in a control chart, which monitored the data and whether all points were within +/- 3 σ of the mean. Figure 2 displays the system suitability results for the resolution between Related Compound B and Related Compound C over the course of the forty days the system was running. All values for the resolution were all within three standard deviations of the mean and the +/- 3 σ of the mean were also within the system suitability requirements of NLT 1.5.
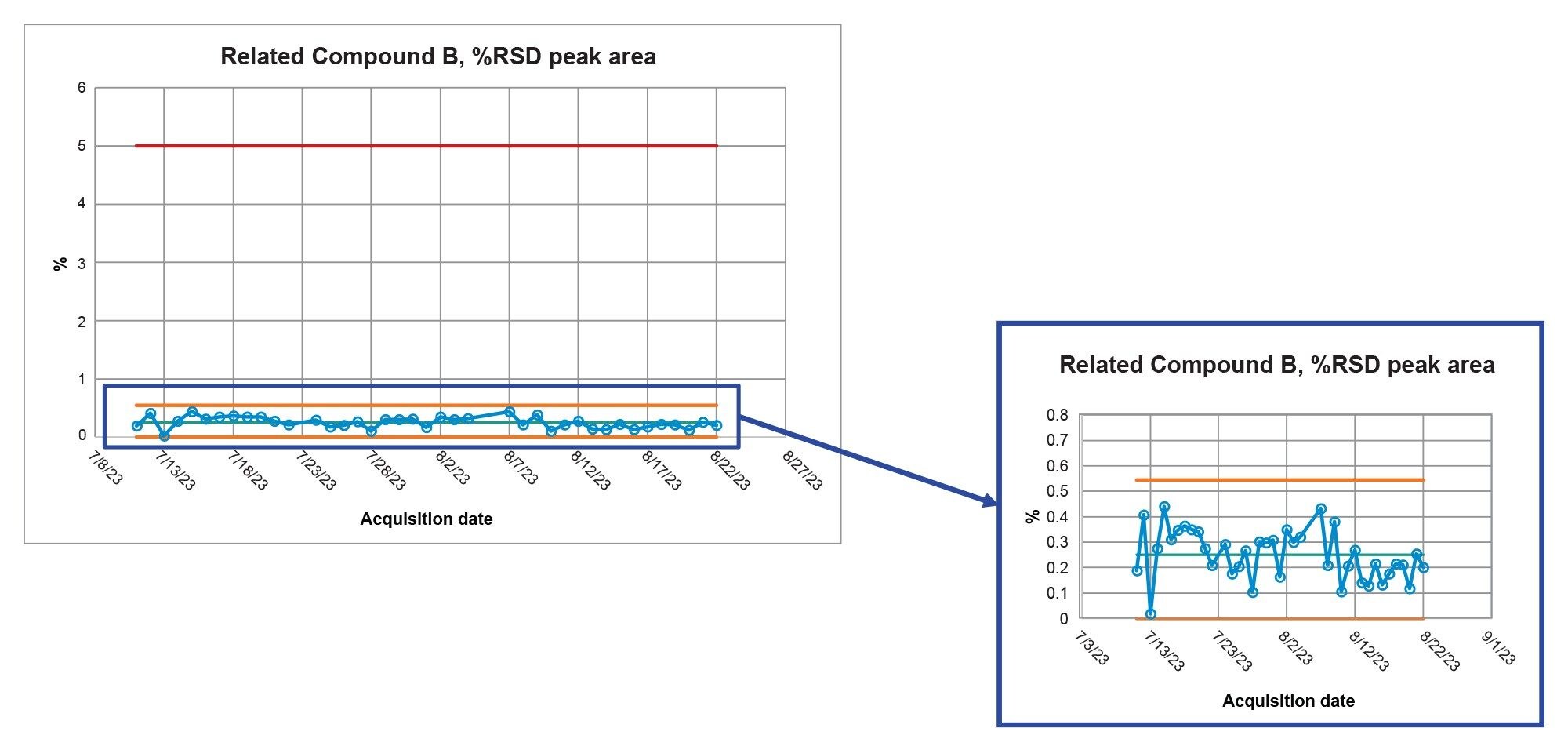
The control chart for the system suitability results for Related Compound A %RSD for Retention Time over the forty days is present in Figure 3. Results for Related Compound A were well below the requirement of NMT 5.0% with all values below 0.3% and in a state of controlled with all the data within +/- 3 σ of the mean. The %RSD for Retention Time results for the remaining compounds, Related Compound B, Related Compound C and Fluconazole, were both well below the requirement of NMT 5.0% and in a control state as depicted in Table 1. The last system suitability requirement is for the %RSD Area for each compound. Figure 4 displays the results for the %RSD Area for Related Compound B in the control chart. The results for all compounds are lower than the requirements with all values below 0.6 %RSD for peak area. With all the results meeting the system suitability requirements and all results within control, the Alliance iS HPLC System demonstrated consistent and reproducible performance throughout all forty days of the study.

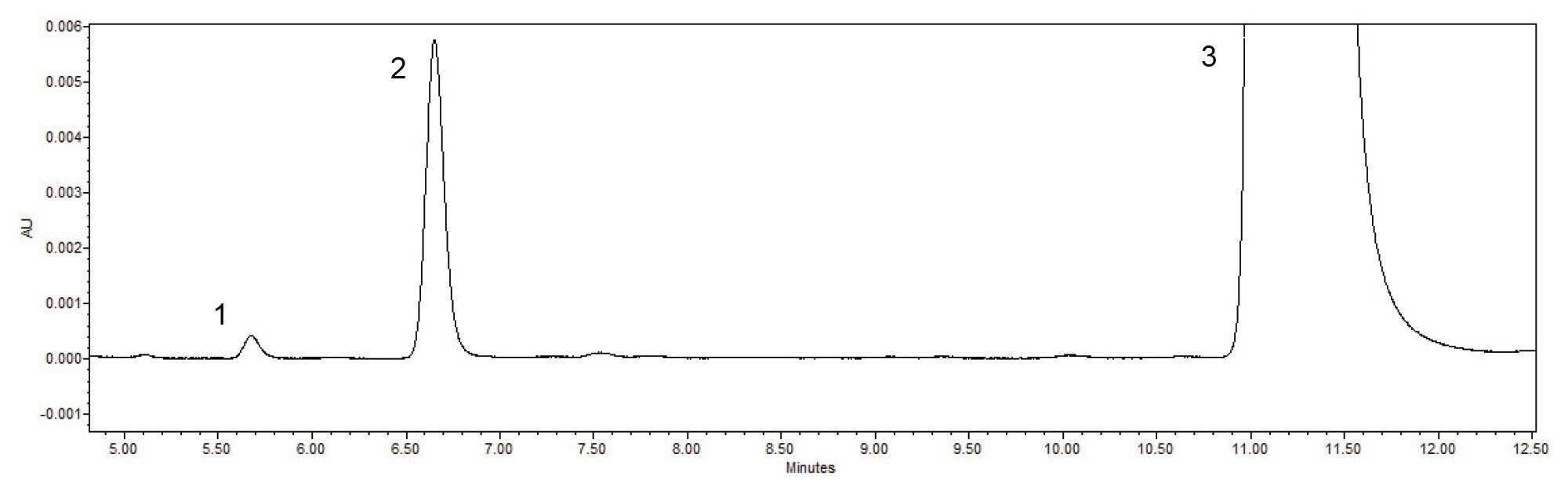
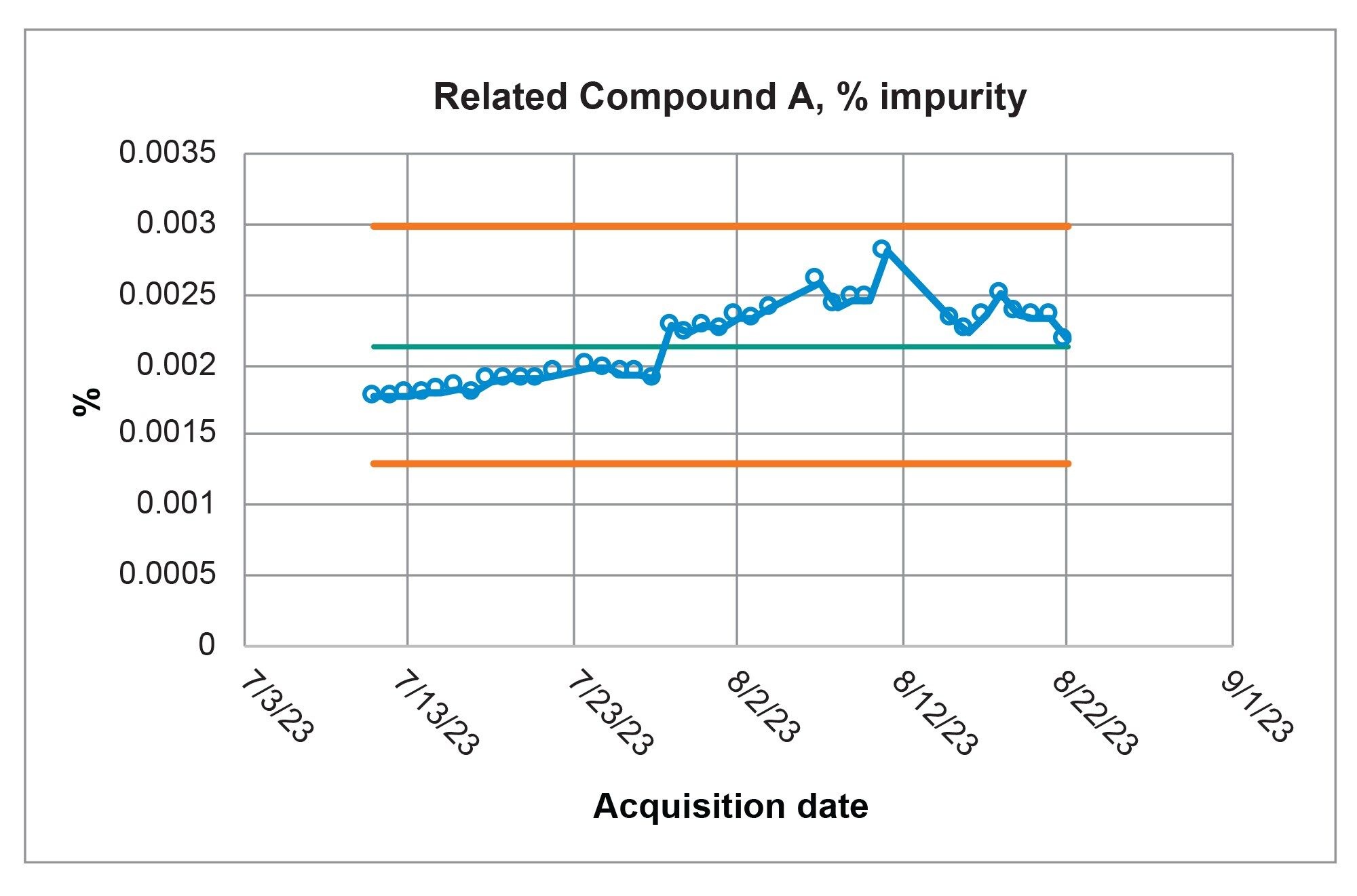
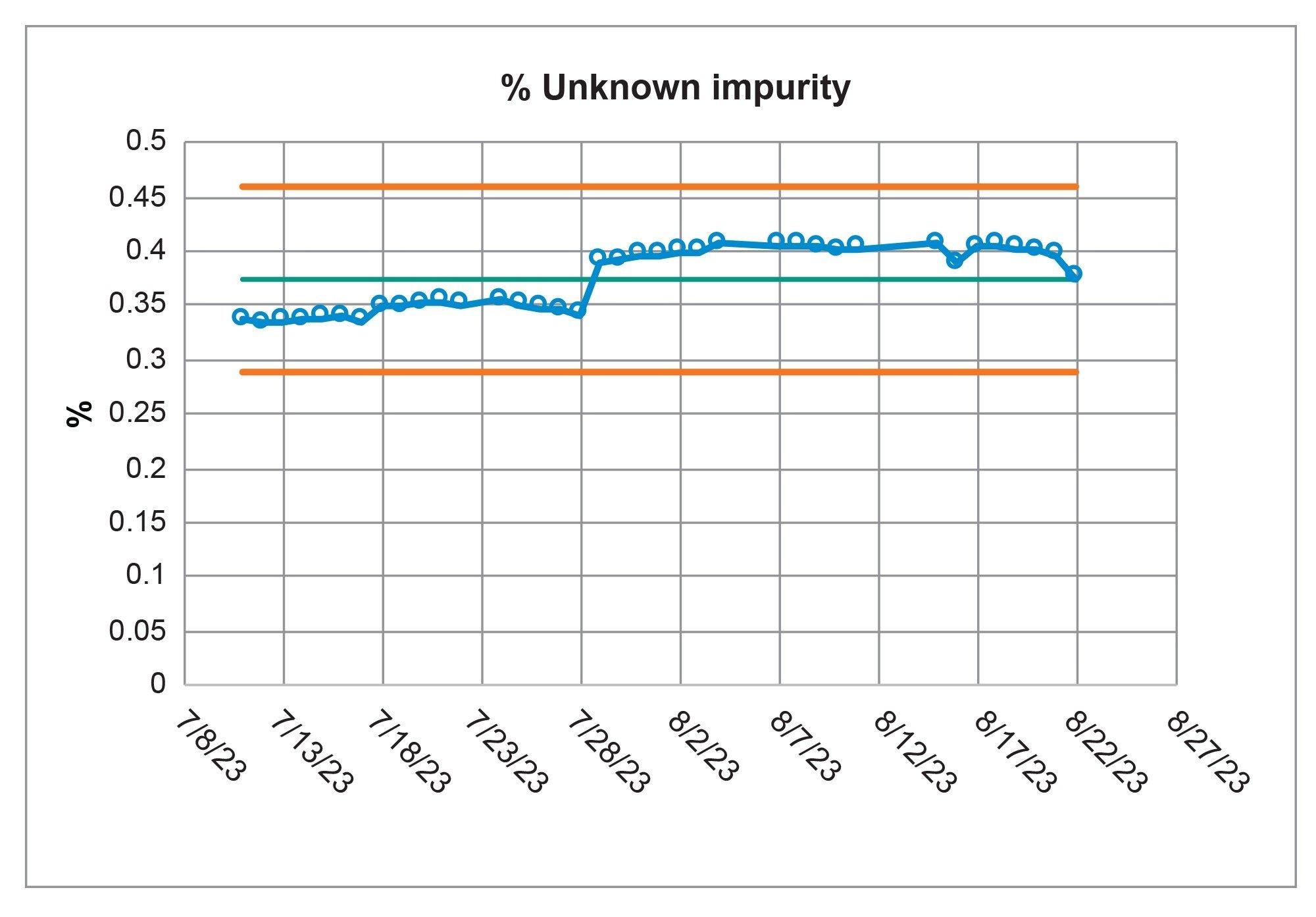
QC laboratories not only need to meet a method’s system suitability requirements, but also need to analyze samples with confidence. With the Alliance iS HPLC System, the system was able to meet the system suitability requirements consistently and analyzed a sample solution reproducibly and reliably over the course of the forty-day study. The Fluconazole sample solution contained two impurities, Related Compound A, and an Unknown Impurity (Figure 5). The quantitative analysis for each of the impurities were calculated based upon the USP monograph.3 Over the forty-day range of the study the average calculated value for the Related Compound A impurity (Figure 6) was 0.002% and the average calculated value for the Unknown Impurity was 0.374% (Figure 7). For each impurity contained in the Sample Solution, all results are recorded in the control chart which demonstrate that the quantitative results were well within the +/- 3 σ of the mean, indicating a state of control of the Alliance iS HPLC System over the full scope of the study.
Conclusion
The USP Fluconazole Organic Impurities method was analyzed on the Alliance iS HPLC System for an extended period of time to demonstrate the robustness and reproducibility of the Alliance iS HPLC System. Over the course of the forty days, the Alliance iS HPLC System executed the USP Fluconazole Impurities method in which the system suitability solution was run every 24 hours along with a sample solution. Throughout the forty-day runtime, the Alliance iS HPLC System demonstrated reproducible system suitability results and reproducible data for the sample solution impurity results. For a QC laboratory, reproducible results are essential to release quality products on time. We have shown that the Alliance iS HPLC System provides the confident and reliable quality results a QC laboratory demands.
References
- USP, Fluconazole. United States Pharmacopeia and Formulary (USP 43-NF38) 2020, (GUID-F961B739-9D37-4719-8E8A-1E7474DFE57D_3_en-US).
- Alliance iS HPLC System
- Zhang X, Birdsall R, You Y, Enhancing Robustness for Biopharmaceutical Separations Using the Waters ACQUITY UPLC PLUS Series. Waters Application Note, 720006285, 2018.
- Francis D, Driving Efficiency in QC Labs. Waters White Paper, 720008074, 2023.
Featured Products
720008254, March 2024