Analytical Quality by Design Based Method Development for the Analysis of Cold and Cough Formulations Using an Arc Premier™ System
Abstract
The separation of active pharmaceutical compounds phenylephrine, acetaminophen, doxylamine succinate, guaifenesin, dextromethorphan, and diphenhydramine was achieved using an Arc™ Premier System with an 11-minute run time while using mass spectroscopy compatible mobile phases and additives. The method development aligns with aspects of the Analytical Quality by Design (AQbD) philosophy described in the ICH Q14 and USP <1220> Guidelines. Development was conducted with the use of Fusion QbD® Software (S-Matrix Corporation). The final reproducible method was successfully tested on four cough syrup formulations with different spike concentrations of the strongly absorbing potassium sorbate excipient.
Benefits
- One method for separation of phenylephrine, acetaminophen, doxylamine succinate, guaifenesin, dextromethorphan, and diphenhydramine, tested on four cough syrup formulations including samples with a spike of the highly absorbing potassium sorbate excipient. Peaks were shown to be spectrally homogenous for all active pharmaceutical ingredients (APIs) present and performance characteristics were maintained
- A fast, reliable, and reproducible method using the Arc Premier System and 2.7 μm CORTECS™ Columns
- Mass Spectroscopy compatible mobile phases, allowing for the use of a mass detector in identification and quantification of peaks
- Aspects of the ICH Q14 Analytical Procedures Development and USP <1220> Analytical Procedure Life Cycle Guidelines incorporated into the method development workflow, assisted by Fusion QbD Software
Introduction
Cough syrup formulations that target the relief of common cold and flu symptoms are found in many different forms and formulations. The APIs added to these medications include decongestants, cough suppressants, expectorants, pain relievers, fever reducers, and/or antihistamines.1 These APIs are administered through various delivery methods such as pressed pills, gel capsules, and syrups. Excipients are used in each delivery method to enhance the quality of the product. In syrup formulations, excipients are used to modify the sweetness, thickness, flavor, color, antimicrobial, and buffering properties of the syrup. The proprietary nature of these formulations results in a market with a wide range of APIs and excipients used in varying concentrations. Around 300 food additives are allowed for use in different drug formulations.2 A study found that there were over a hundred different excipients present in 60 cough syrup formulations.3 These excipients can cause problematic matrix effects and coelutions, making the analysis of formulations challenging.1
HPLC is commonly used for analyzing cough syrups because it can separate and quantify APIs and excipients with high sensitivity and selectivity.4 Currently, the industry uses separate chromatographic methods to analyze each API in pharmaceutical formulations. While effective, this approach can be very time consuming and generates large amounts of hazardous organic solvent waste. To make such analyses more efficient, one solution is to use a single chromatographic method to analyze multiple APIs in various pharmaceutical formulations.
AQbD is a systematic approach to analytical method development that aims to ensure quality, in part by identifying and controlling sources of variability throughout the method's lifecycle. AQbD principles are becoming increasingly important in the pharmaceutical industry, where regulatory agencies such as the USP and ICH are emphasizing the need for quality assurance and control. AQbD involves the use of statistical tools, design of experiments (DOE), risk assessments, and knowledge management to enhance method robustness, reliability, and consistency. By incorporating AQbD principles, analysts can reduce the time and cost of method development, while improving the overall quality of analytical results.
Software tools can be used to implement AQbD principles in analytical method development by assisting users in designing and executing experiments, analyzing data, and optimizing methods. The software helps in identifying the critical method parameters (CMPs) and the critical method attributes (CMAs) for a given method. It also helps in determining the relationships between the CMPs and CMAs, allowing users to identify the most important factors that affect the quality of the method.
In the work shown here we developed a UHPLC method for separating six APIs in cough syrup formulations. The method was tested on four formulations and was able to maintain resolution and peak purity of present APIs in each formulation while in the presence of a 100% potassium sorbate spike.
Experimental
Sample Preparation
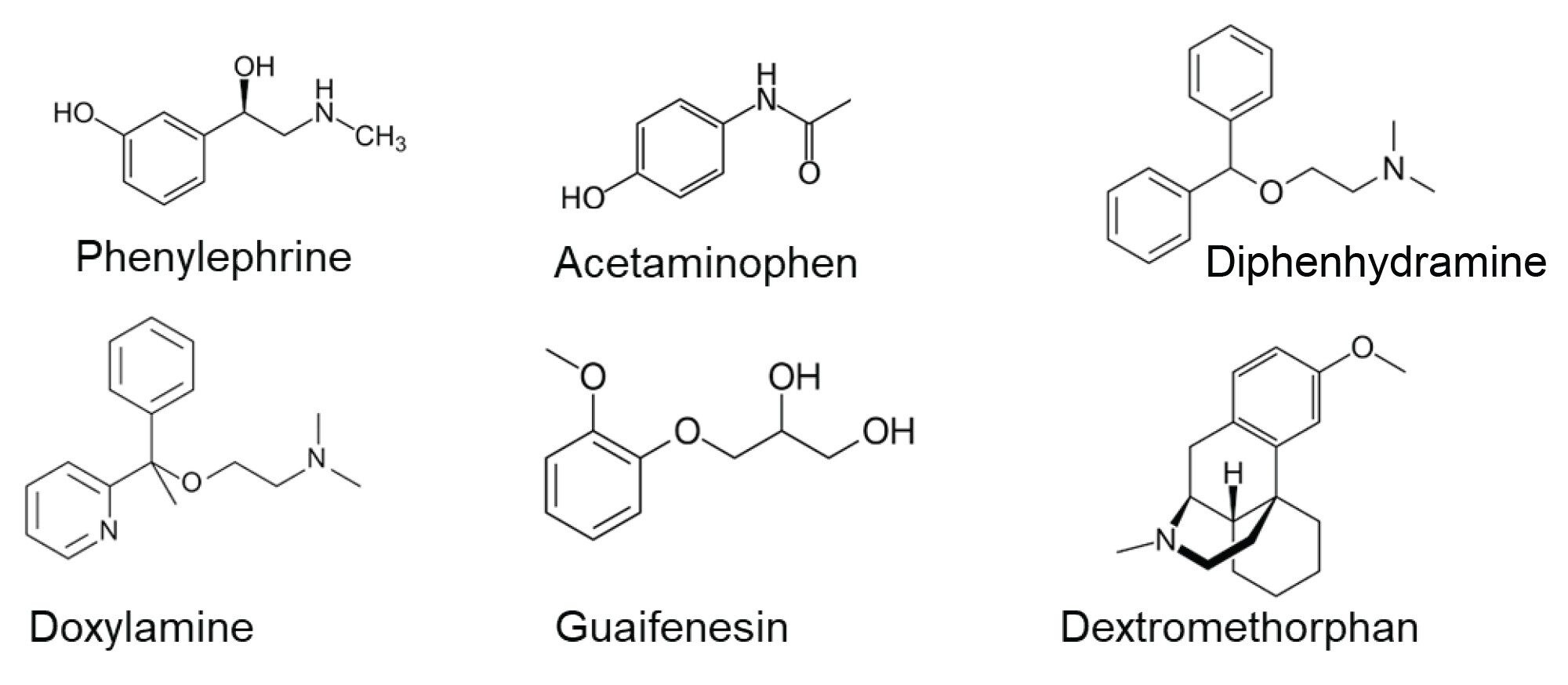
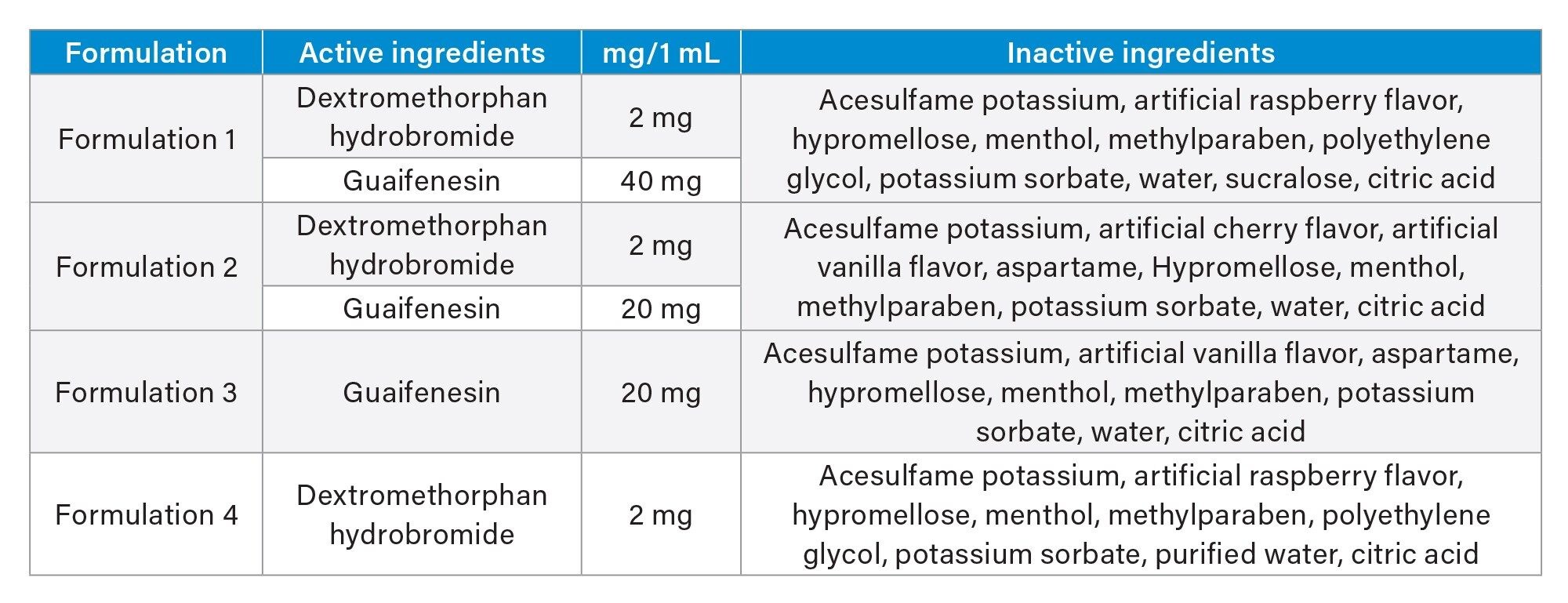
A standard solution was prepared using 1 μg/mL concentrations of phenylephrine, acetaminophen, doxylamine succinate, guaifenesin, dextromethorphan, and diphenhydramine, dissolved in DI water. The chemical structures of these six APIs can be seen in Figure 1. Formulations were purchased from an online pharmacy, and their contents are listed in Table 1. To prepare the samples for analysis, the formulations were diluted 1000-fold in DI water. Spike samples were also prepared by diluting them 1000-fold in DI water and spiking them with three different concentrations of potassium sorbate 20%, 50%, and 100%. With 100% being double the amount of potassium sorbate found in the formulations.
Screened LC Conditions:
|
Column(s): |
1. CORTECS Premier C18 2.7 μm 2.1 X 100mm 2. CORTECS Premier C18+ 2.7 μm 2.1 X 100mm 3. CORTECS Premier T3 2.7 μm 2.1 X 100mm 4. CORTECS Premier Phenyl 2.7 μm 2.1 X 100mm |
|
Column temp.: |
30–45 °C |
|
Flow rate: |
0.6 mL/min |
|
Mobile phase A: |
0.1% Formic Acid in DI Water |
|
Mobile phase B: |
0.1% Formic Acid in Acetonitrile |
|
Gradient: |
3% to 70% Acetonitrile in 3–15 minutes |
Final LC Conditions:
|
LC system: |
Arc Premier System Quaternary Solvent Manager Sample Manager FTN-R Column Manager with Auxiliary Unit |
|
Detection: |
PDA 2998 ACQUITY QDa™ Mass Detector |
|
Vials: |
2 mL TrueView |
|
Column(s): |
CORTECS Premier T3 2.7 μm 2.1 X 100mm |
|
Column temp.: |
45 °C |
|
Sample temp.: |
20 °C |
|
Injection volume: |
10 μL |
|
Flow rate: |
0.6 mL/min |
|
Mobile phase A: |
0.1% Formic Acid in DI Water |
|
Mobile phase B: |
0.1% Formic Acid in Acetonitrile |
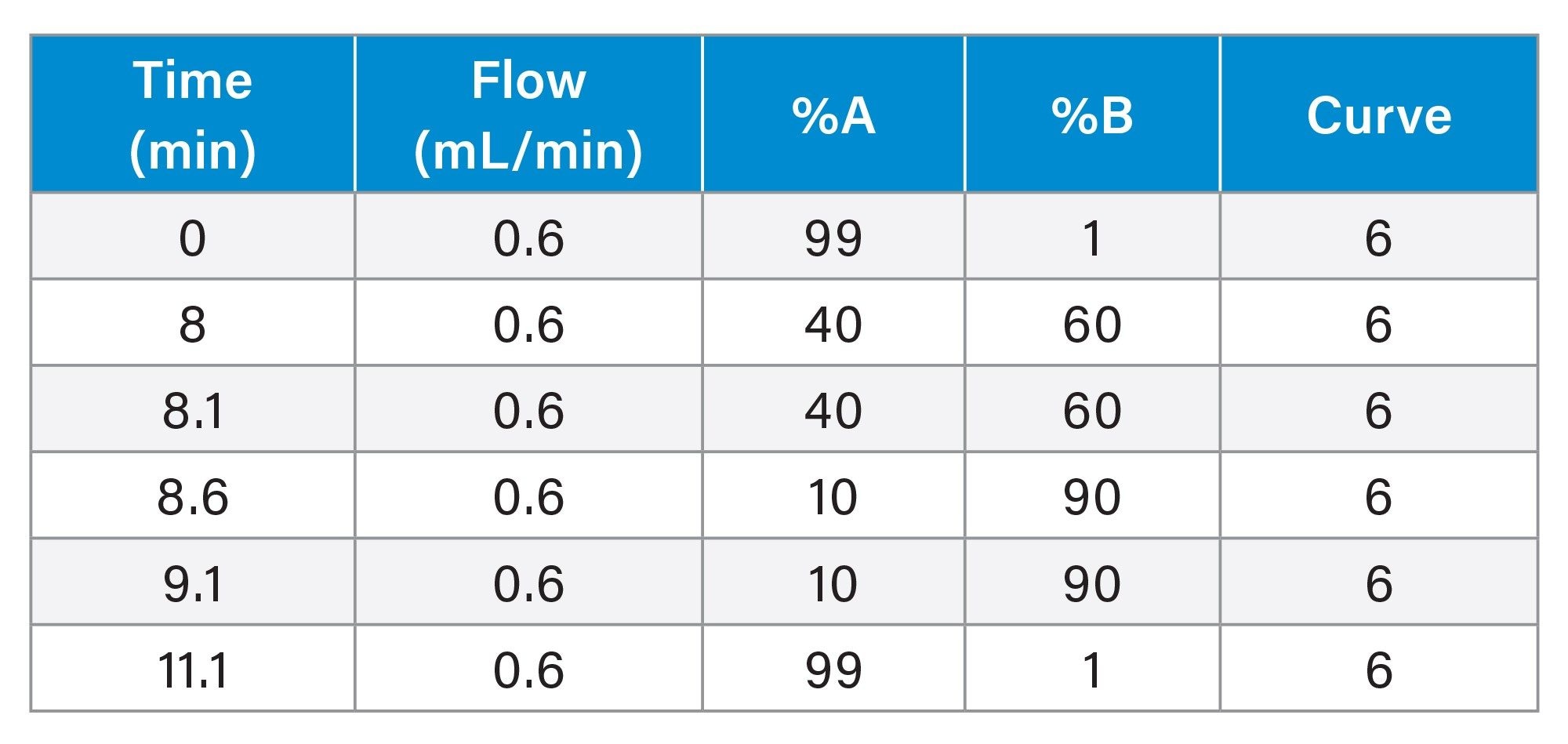
Gradient Table:
Data Management
|
Chromatography software: |
Empower™ Software 3.6.1 |
|
Informatics: |
Fusion QbD 9.9.1.b Build 334 |
Results and Discussion
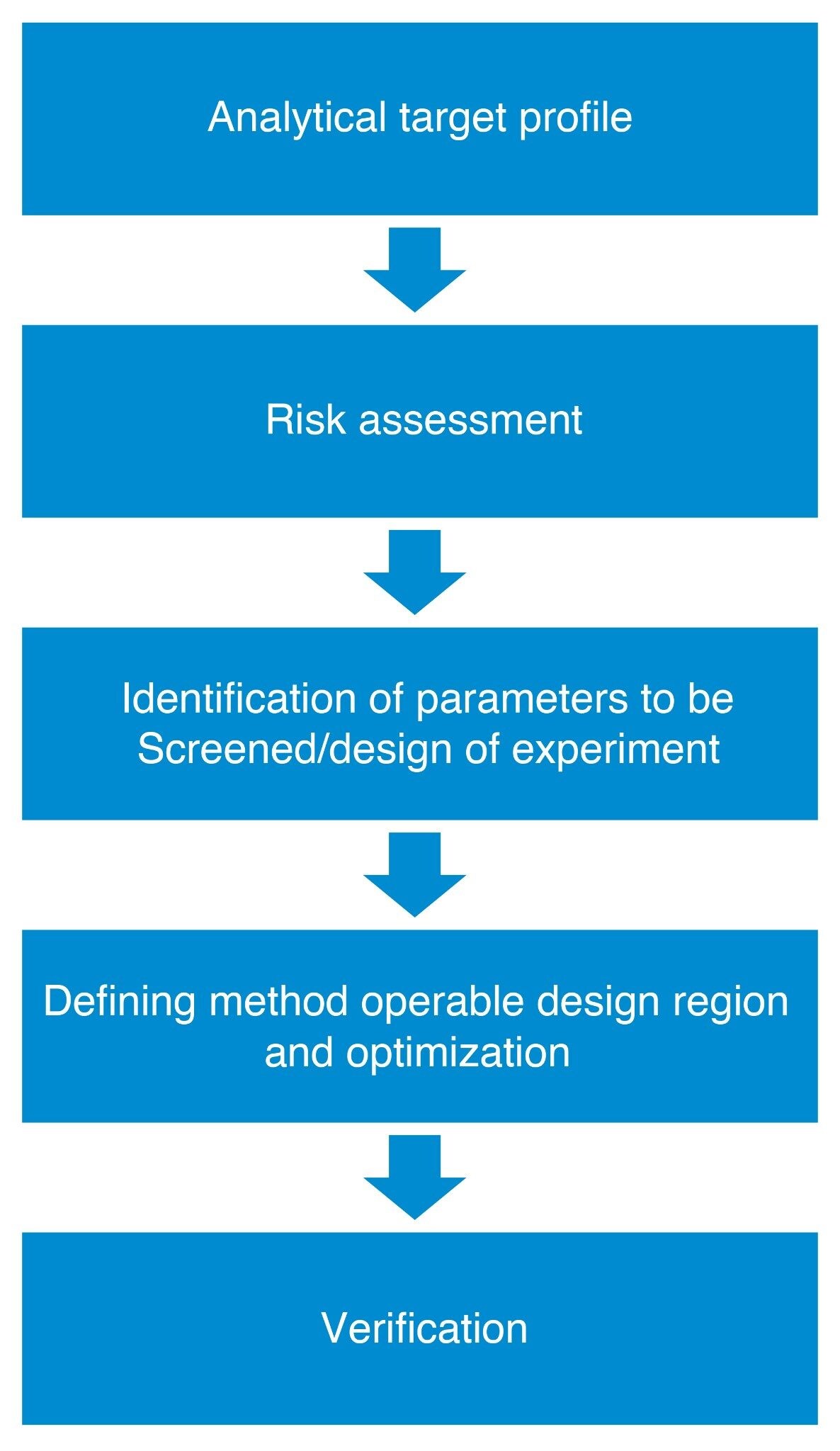
The method development workflow deployed in this study involved multiple steps that were in alignment with the AQbD principles as regulatory agencies have outlined in their papers USP <1220> and ICH Q14.5,6 The steps included determining the analytical target profile (ATP), risk assessment, creation of a design of experiment, determining the method operable design region, optimization, and verification. Using this workflow, a quality method was produced.
Analytical Target Profile
The Analytical Target Profile (ATP) is a crucial element of the AQbD approach for developing analytical methods. It encompasses a specific set of measurable characteristics that an analytical method must meet to ensure it is suitable for its intended purpose. The ATP consists of critical analyte attributes and performance characteristics, including but not limited to bias, precision, specificity, limit of detection, limit of quantitation, linearity, range, ruggedness, and robustness. By providing guidance for the development and optimization of the analytical method, the ATP ensures that the method meets predefined criteria, resulting in a fit-for-purpose method.
In this study, the ATP aimed to develop a precise method capable of separating APIs from excipients present in different cold/cough syrup formulations while obtaining an acceptable limit of detection and quantification. Less than 1% relative standard deviation of peak retention time and peak area for all analytes was desired to demonstrate accuracy and precision. The method was required to maintain performance in the test formulations with a large spike of the excipient potassium sorbate.
The study began by selecting an appropriate technology, which based on previous knowledge was determined to be an Arc Premier System equipped with a Quaternary Solvent Manager (QSM), a Column Manager (CM), and a solvent select valve to enable automated exploration of a wide range of conditions. A QDa Mass Detector was also employed to assist with method development and peak identification. Maintaining the method to be compatible with mass spectrometry was important in this study, as certain analytes have low UV absorbance, which could enable quantification through mass detectors. The solid core technology of the CORTECS Columns was determined to be beneficial due to the QA/QC use case of such a method, where a higher pressure and shorter run time may be beneficial.
Risk Assessment
During this phase of the study, we thoroughly evaluated high-risk parameters that could potentially impact the quality of the data generated by the method, as well as its ability to achieve its goals. This evaluation is based on sound chromatographic principles, prior knowledge, and expertise.
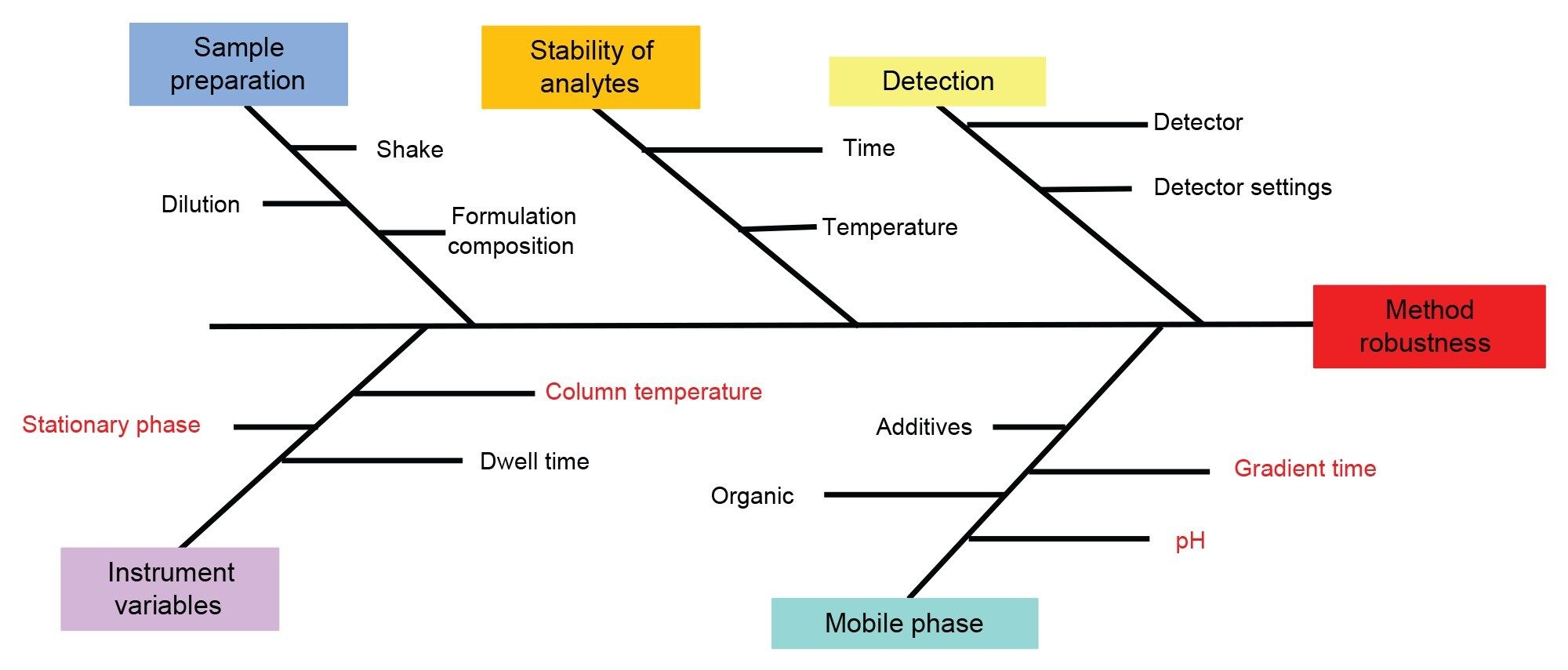
Given the significant impact that column stationary phase has on compound separation, it was deemed a high-risk factor in this study. Therefore, four distinct stationary phase chemistries were chosen to be screened to ensure a broad range of selectivity and maximize the probability of successfully resolving all analytes. The pH was also hypothesized to have a major impact on the performance of the analytical method. Consequently, preliminary experiments were conducted over a broad pH range, revealing that a lower pH was optimal for most analytes. Gradient time and column temperature were both considered to be high risks as variations could cause coelution in the formulations. As such it was determined that these two factors need to be investigated in depth in subsequent steps of the method development process. It was hypothesized that sample preparation would pose a low risk for both standards and formulations, given that all samples were water-soluble, making it easier to extract target analytes and reducing the risk of strong solvent effects. Stability was found to be a relatively low risk factor as the formulations are shelf stable. As with all methods detection needs to be carefully considered. Mass spec was used for peak identification during method development but could also be used for the quantification of some analytes. The chemicals found in these formulations vary drastically in their chemical properties, thus no UV wavelength was best for all analytes so a standard 254 nm was used. The detection method and parameters will affect the LOD and LOQ but not the retention time. These variables were characterized as low risk for the method as the ATP was focused on separation not low detection limits. To illustrate all the method parameters and their respective impact, we have included a fishbone diagram in Figure 3.
Design of Experiment
At this phase of the study, a DOE was devised based on the critical method attributes and risk factors identified during the risk assessment. The objective was to thoroughly explore the impacts of column chemistry, gradient time, and temperature on the process. After selecting all the variables and the constants for the experiment in Fusion QbD, the software created an experimental design consisting of thirty-two runs using statistical sampling. The software automatically created all the required instrument methods and equilibration/conditioning steps within the Empower Software project.
Design of Experiment
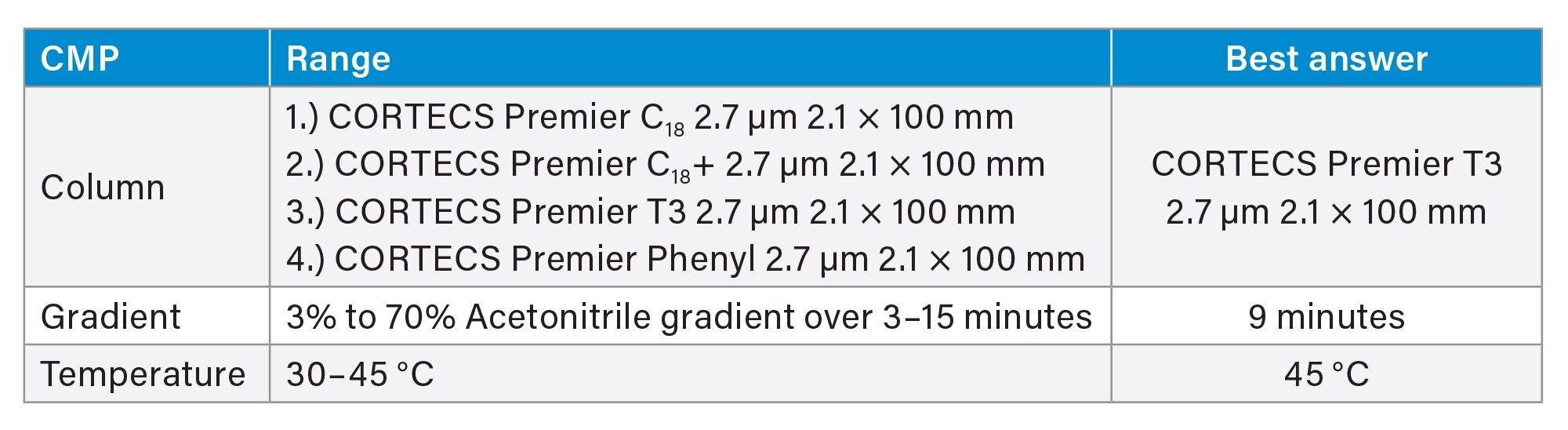
The next step was processing the data in the Empower CDS and importing them into Fusion QbD Software to find the best overall answer (BOA) where performance goals are met. The performance goals that were set in this screening stage included the maximum number of peaks with a resolution ≥4 and the maximum number of peaks with a USP tailing factor of ≤1.5. These goals were set after visual examination of the screening experiment chromatograms. Processing the data provided a simulation with a model sufficiency of 0.999 that revealed the best combination of conditions predicted to achieve the set performance criteria was a CORTECS Premier T3 Column, a temperature of 45 °C and gradient time of 9 minutes. Figure 4 Shows the MODR where the white areas represent methods that satisfy the set performance characteristics.

Using the previous knowledge and information gained during the risk assessment stage, further optimization of the best overall answer was conducted to improve the method’s performance. Optimization of the BOA was accomplished without Fusion QbD Software. Three changes were made to improve the chromatographic properties of the method, allowing for a better separation of formulations. No peaks eluted in the last minute of the gradient. To reduce wasted solvent and reduce the run time the method’s maximum acetonitrile percentage was decreased to 60% and the gradient was reduced to 8 minutes, thus approximately maintaining the gradient slope. The first eluting peak, phenylephrine, was seen eluting too close to the void time so the gradient starting point was reduced to 1% acetonitrile.
Verification
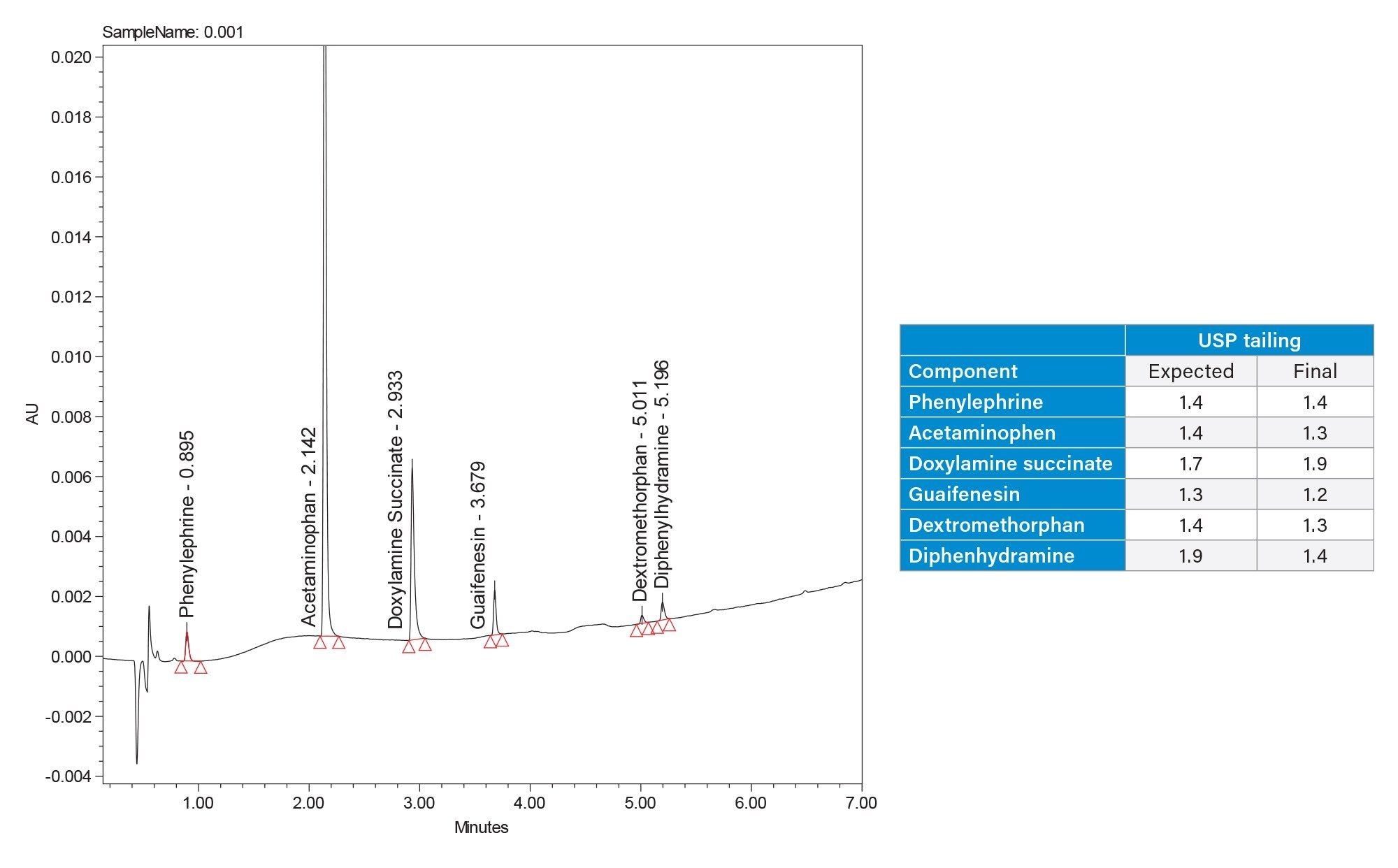
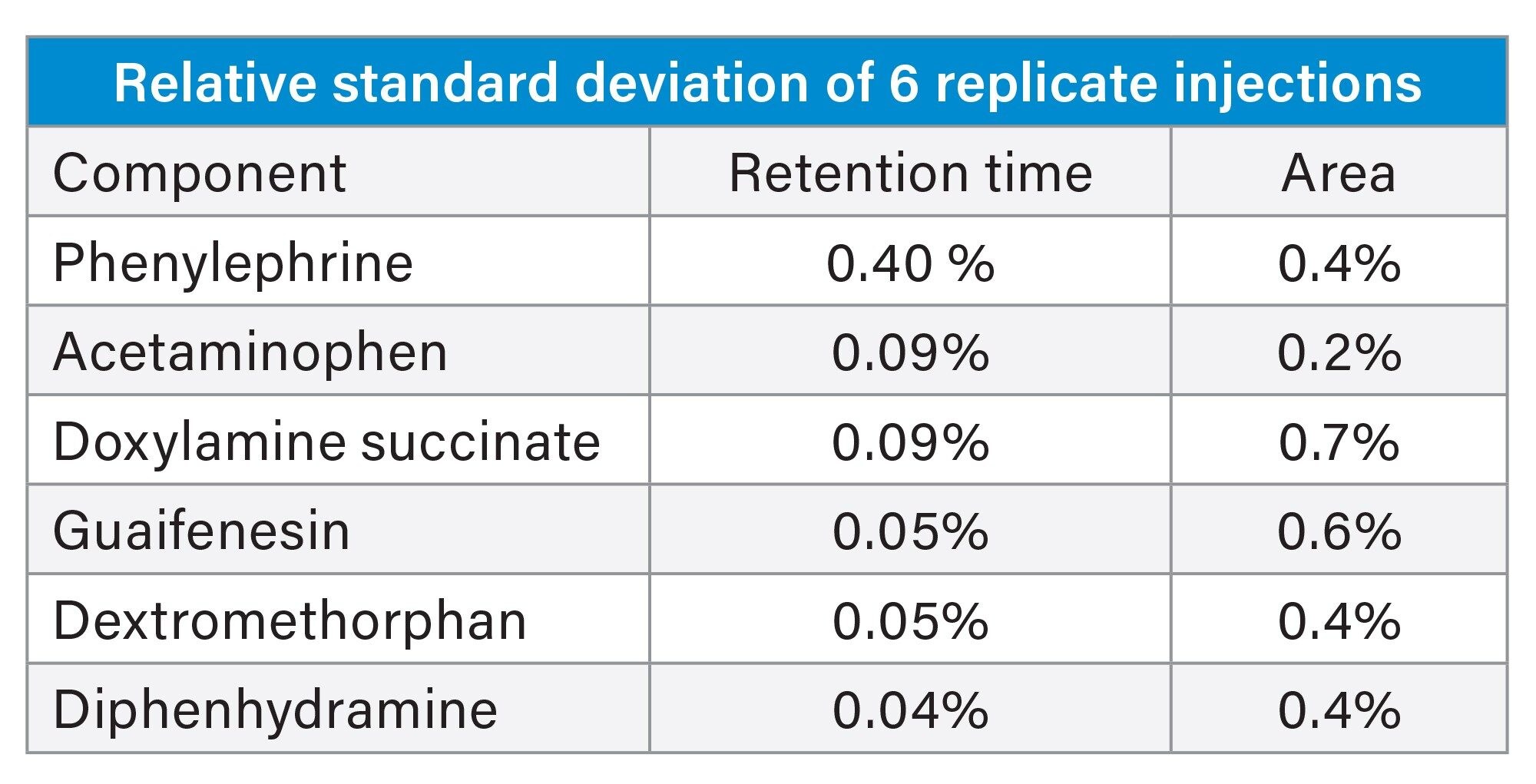
The final separation of the 6 APIs can be seen in Figure 5. The method performance goals were met for all analytes except doxylamine succinate, which was seen to have substantial tailing. Although not ideal this tailing did not reduce the methods reproducibility below an acceptable level. Reproducibility results can be found in Table 3; the relative standard deviations of the retention time and the area suggests a reproducible method. These results accomplish the goals set in the ATP.
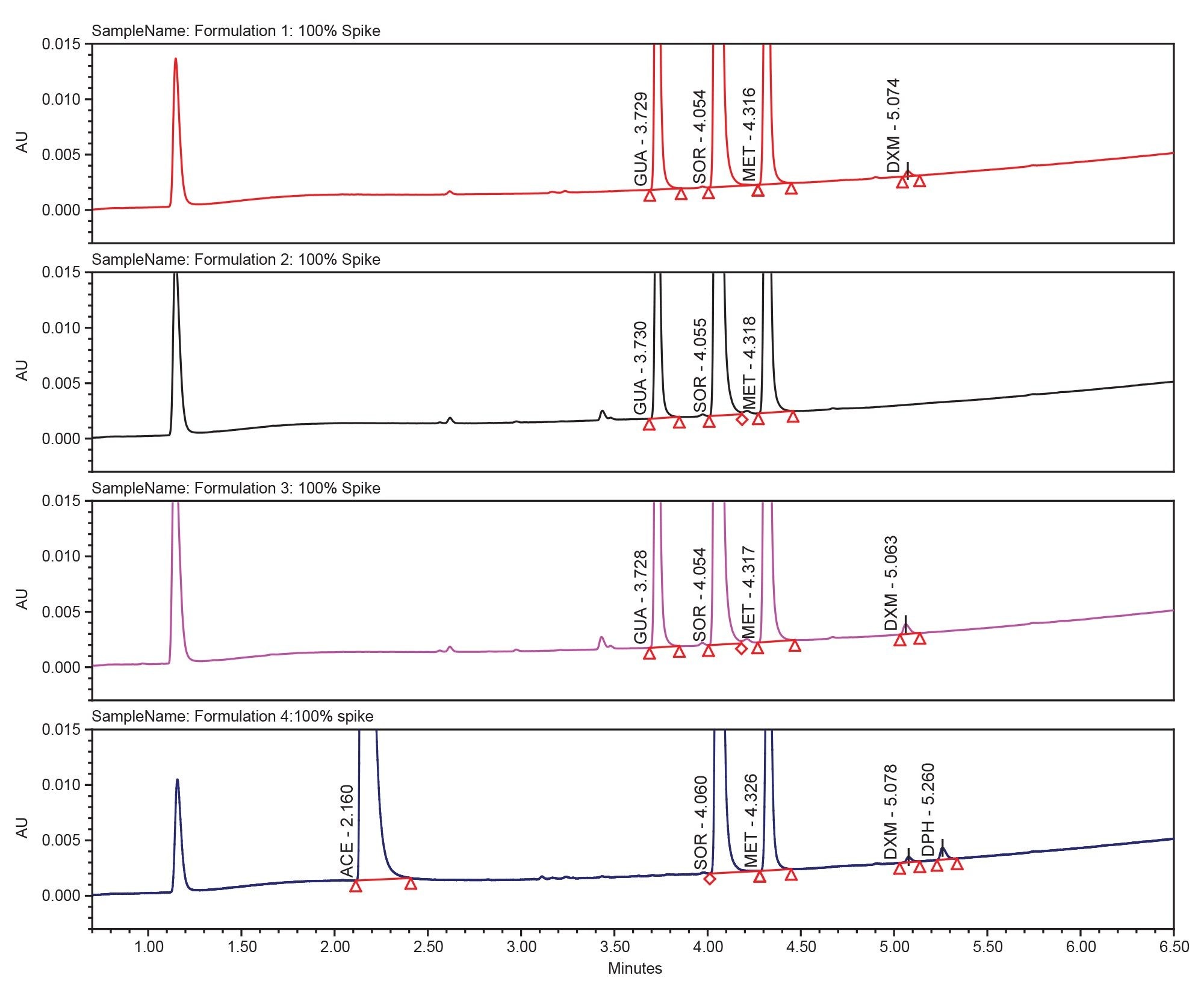
As part of the method verification and assessment the limit of detection (LOD) and limit of quantitation (LOQ) for the 6 APIs were assessed using the USP <621> guidelines.7 Noise was calculated using a blank sample and the latest USP guidelines. The wavelength of 254 nm was used for calculations, lower LOD and LOQ could likely be obtained using different wavelengths or different detectors as no single method of detection is optimal for all APIs. LOD and LOQ concentrations were found to be acceptable.
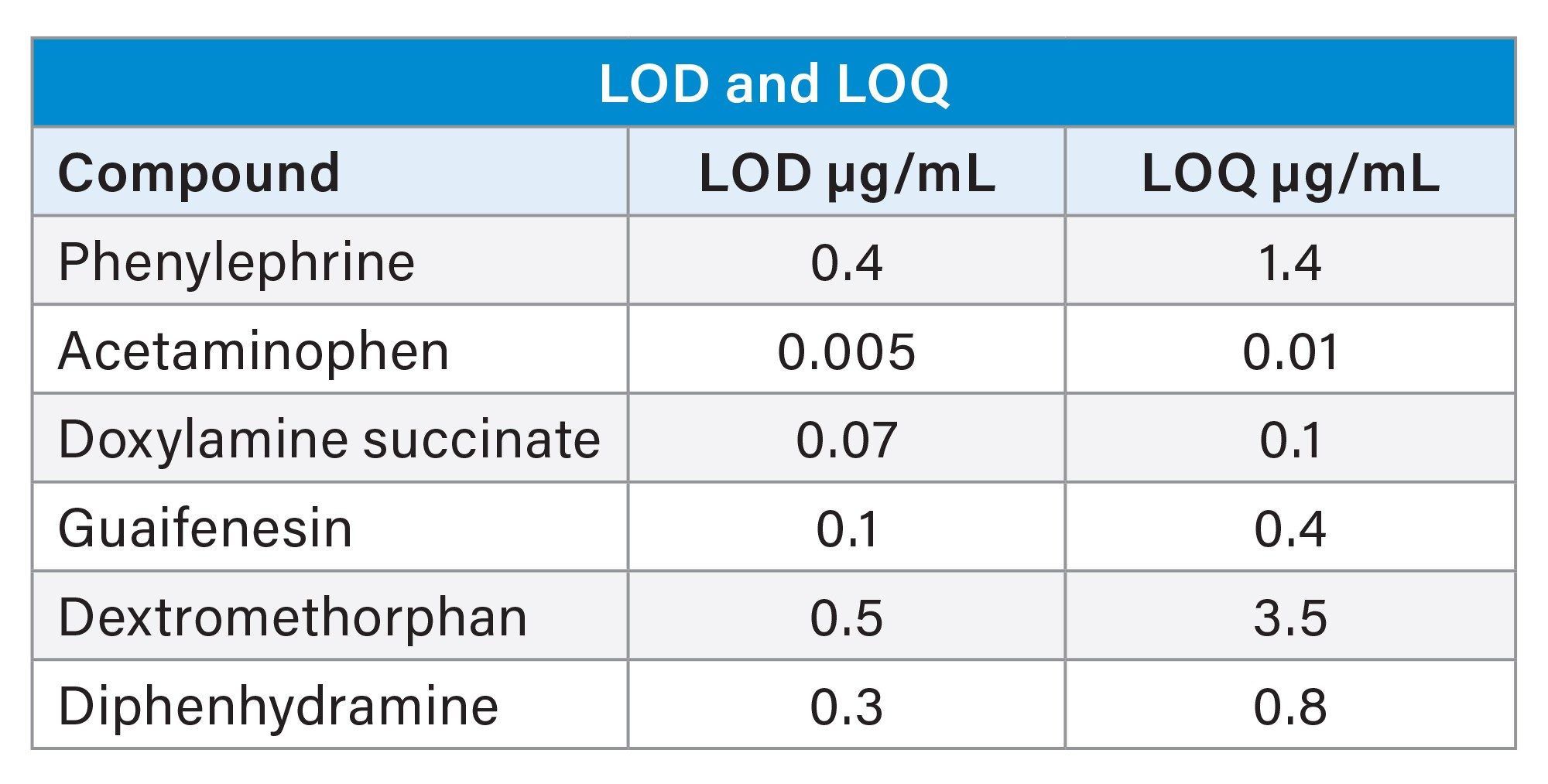
Method Application: Pharmaceutical Formulations, Excipient Spikes and Peak Purity
In this phase of the study, our objective was to evaluate the performance of the developed method by analyzing pharmaceutical formulations of cough syrup. Additionally, we sought to assess the method's robustness by determining its ability to accurately detect the presence of varying concentrations of one of the excipients in the samples. Potassium sorbate was identified as an excipient of interest due to its frequent usage in such formulations.8 This antibacterial and antifungal excipient needs to be monitored closely due to its potentially poisonous and carcinogenic effects associated with long term use.9 Additionally, its strong UV absorbance makes it a good candidate for the development of an HPLC-UV method.
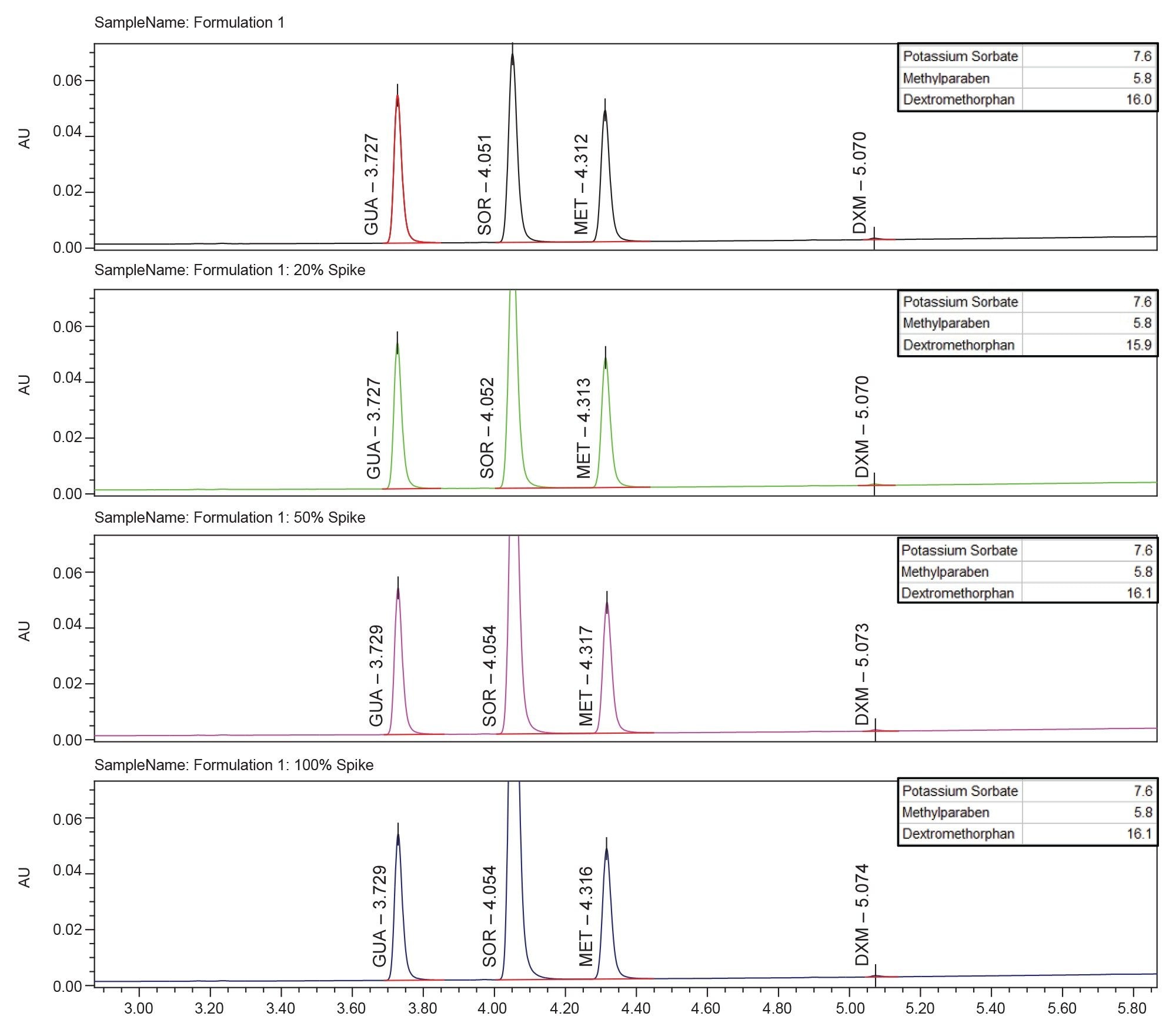
The final method was tested on all four formulations at four concentrations of potassium sorbate each including: no spike, 20%, 50%, and 100% spike. All formulations at all concentrations were found to have acceptable resolution and chromatographic characteristics. Example chromatograms of each spike level are seen for the formulation 1 in Figure 6. Figure 7 shows the 100% spike of potassium sorbate for all formulations. Resolution of all APIs including the critical pair did not deteriorate at any potassium sorbate spike concentration for all formulations. The retention time, area, and tailing of all APIs were also not significantly changed in any formulation at any spike level.
Peak purity testing is important for any method but especially when working with formulations that contain a wide range of excipients, as coelutions will cause inaccurate results. Avoiding these coelutions is difficult due to the number of unknown peaks in these cough syrups. Peak purity testing was completed using UV analysis.10 APIs and the excipients potassium sorbate and methylparaben were tested. All purity angles were found to be less than the purity threshold demonstrating spectral homogeneity which suggests peak purity.
Conclusion
A single method for separation of phenylephrine, acetaminophen, doxylamine succinate, guaifenesin, dextromethorphan, and diphenhydramine was developed. This obtained the desired chromatographic properties for all analytes except doxylamine succinate which was found to have more tailing than desired, but did not affect the methods reproducibility below an acceptable level. The method was able to separate all APIs from the excipients found in a selection of common cough syrups. Peak purity was tested using data from the PDA and Empower Software’s UV peak purity testing tool. Results showed spectral homogeneity of all APIs in the tested samples. Resolution was unaffected in all formulations at all potassium sorbate spike concentrations.
An Arc Premier System equipped with a Quaternary Solvent Manager (QSM), a Column Manager (CM) and a solvent select valve. to enable automated exploration of a wide range of conditions, was employed. The reliable Arc Premier System and the CORTECS Columns allowed for a fast separation of the API compounds. This was achieved while keeping the mobile phases mass spectrometer compatible to allow for the use of a QDa Mass Detector to assist with method development and peak identification.
AQbD principles and the benefits of enhanced approaches to method development have gained greater acceptance with the publication of the USP <1220> Analytical Procedure Life Cycle and ICH Q14 Analytical Procedure Development Guidelines. Fusion QbD Software assists users in designing and executing experiments, analyzing data, and optimizing methods. The software helps in determining the relationships between the CMPs and CMAs, allowing users to identify the most important factors that affect the quality of the method. Incorporating AQbD principles into the method development workflow outlined by these documents aided in creating a quality method.
References
- Özdemir A, Aksoy H, Dinç E, Băleanud D, Dermişc S. Determination Of Guaifenesin And Dextromethorphan In A Cough Syrup By Hplc With Fluorometric Detection. Revue Roumaine de Chimie. 2006;51(2): 117–122.
- Dinç-Zor Ş, Dönmez ÖA, Aşçı B, Pingo E. Chemometric Optimization of an Hplc Method for the Simultaneous Analysis of a Multi Component Drug Product by the Help of Central Composite Design. Microchemical Journal. 2020;152(152).
- Eccles R. What is the Role of Over 100 Excipients in Over the Counter (OTC) Cough Medicines? Lung Journal. 2020;198: 727–734.
- Maziarz M, Rainville P. Robust and Rapid Method Development for Analysis of Active Pharmaceutical Ingredients in Multi-Component Cold and Flu Medication. Waters Application Note. 720006523.
- ICH Harmonized Guidelines, Analytical Procedure Development Q14, March 2022.
- USP General Chapter, USP-NF <1220> Analytical Procedure Life Cycle, The United States Pharmacopeia Convention, Official May 2022.
- USP Stage 4 Harmonization, USP-NF <621>Chromatography, The United States Pharmacopeia Convention, Official December 2022.
- Yuliana T, Gustin SSN, Alamsyah A, Budiman S, Hardian A, Yun YF, et al. HPLC Method for Simultaneous Determination of Dextromethorphan Hydrobromide, Chlorpheniramine Maleate and Potassium Sorbate in Cough Syrup. IOP Conference Series: Materials Science and Engineering. 2021;1115(1).
- Grumbach E, Diehl D, Mazzeo J. Quantitation of Over the Counter Cold Medicine Formulations using UPLC Technology. Waters Application Note. 720002005. 2007.
- Maziarz M. Verifying Spectral Purity of a Chromatographic Peak Using Empower CDS Software. Waters Application Note. 720006582. 2019.
720007957, July 2023