Temperature Dependence on Reversed-Phase Separations of Fatty Acid Modified GLP-1 Receptor Agonists and Their Impurities
Abstract
Here, we show that the reversed-phase separation of fatty acid modified GLP-1 RAs and their impurities is highly dependent on column temperature. The temperature dependence of these separations can be used to modulate and optimize resolution of critical impurity species. These results also emphasize that strict temperature control for these separations is essential, and capability should be considered when selecting instrumentation for reversed phase separation of fatty acid modified GLP1-RAs.
Benefits
- A method to modulate the resolution of GLP-1 RA impurity species during reversed phase separations
- Insights into the importance of tightly controlling column and mobile phase temperatures for the reversed-phase analysis of fatty-acid modified GLP-1 RAs
Introduction
Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are a class of biotherapeutic peptide-like drugs developed to treat Type 2 diabetes. The recent FDA approval of multiple GLP-1 RAs for weight loss has led to rapid increase in their demand and use.1 The prevalence of these drugs prompts a need for robust chromatographic methods to screen for impurities that may impact their safety and efficacy.
Waters™ recently published a single RPLC-UV/MS method for the analysis of a series of GLP-1 RAs using a systematic protocol for method development.2 Further expansion of this work provides analyte-specific tailoring for the development of RPLC-TUV methods for fatty acid modified GLP-1 RAs.3 We demonstrated that minor differences in the chemical structure of fatty acid modified GLP-1 RAs strongly impact their reversed phase impurity separation and suggest that column selection for RPLC-UV impurity analysis of fatty acid modified GLP-1 RAs should be specifically tailored to the analyte of interest.
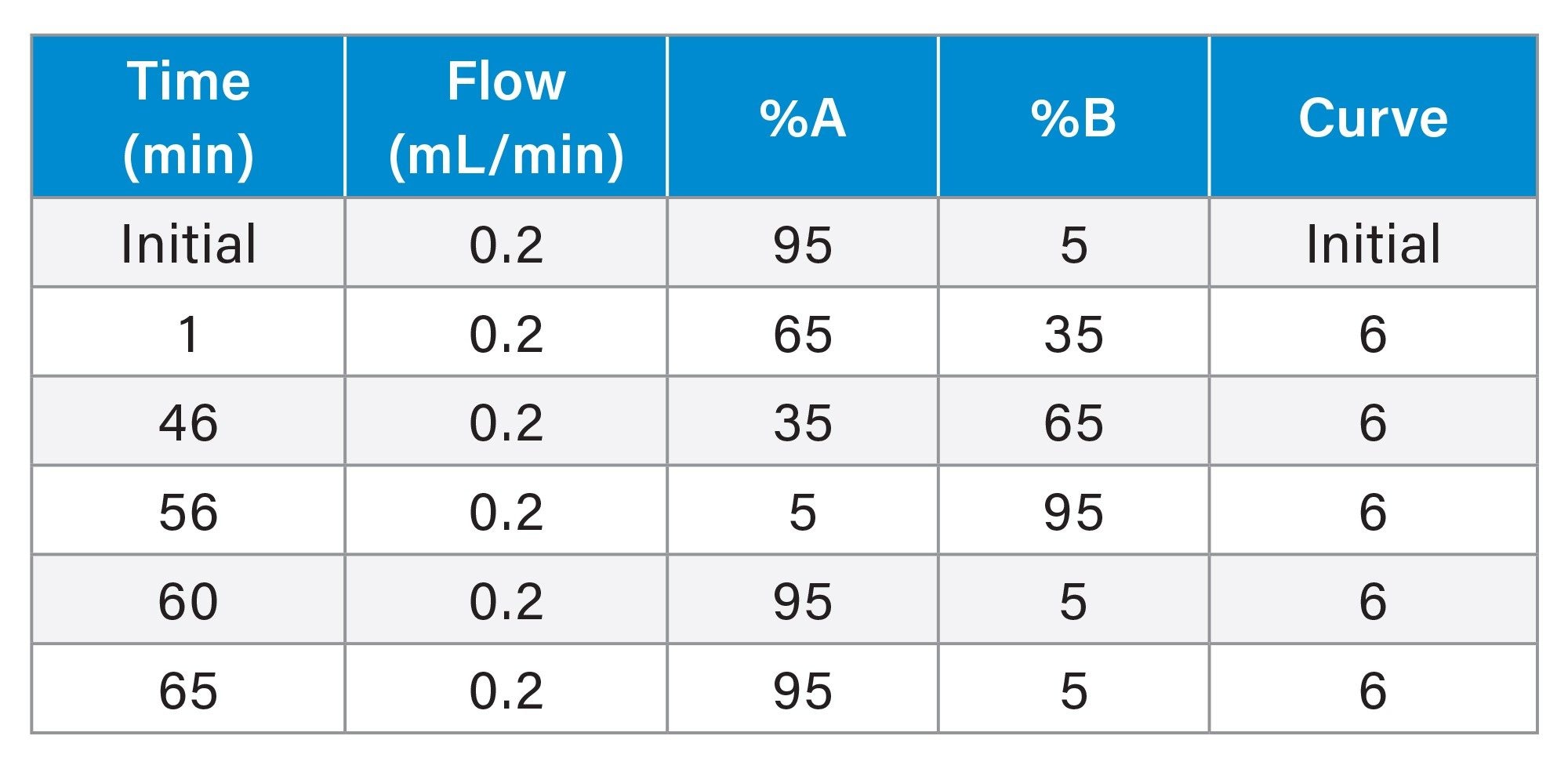
Here, RPLC-UV of liraglutide, tirzepatide, and semaglutide was performed on an ACQUITY™ Premier Peptide CSH™ C18, 1.7 µm Column at 55, 60, and 65 °C. Small changes in column temperature impact the selectivity and resolution of impurity species for all three analytes. We suggest that the temperature dependence of these separations can be used to modulate and optimize resolution of critical impurity species. In addition, the ability of the LC instrumentation to provide effective column and solvent temperature control is critical for reliable reversed-phase separation of fatty acid modified GLP-1 RAs.
Experimental
Sample Description
Liraglutide (AA Blocks, lot no. 4437422), tirzepatide (AA Blocks, lot no. 3620254), and semaglutide (AA Blocks, lot no. 8699981) were reconstituted at 1 mg/mL in a 3:1 mixture of 0.1% TFA in H2O:DMSO.
LC Conditions
|
LC system: |
ACQUITY™ Premier UPLC™ |
|
Column: |
ACQUITY Premier Peptide CSH™ C18, 130 Å, 1.7 µm, 2.1 x 150 mm (p/n: 186009489) |
|
Column temperature: |
55, 60, or 65 °C |
|
Sample temperature: |
6 °C |
|
Injection volume: |
5 µL |
|
Mobile phase A: |
0.1% TFA in H2O |
|
Mobile phase B: |
0.1% TFA in ACN |
|
Detection λ: |
214 nm |
|
Sample vials: |
QuanRecovery™ MaxPeak™ 12 x 32 mm Propylene 300 µL Screw Cap vials (p/n: 186009186) |
Liraglutide and Tirzepatide
Semaglutide

Results and Discussion
Figure 1 shows the chromatographic results for liraglutide, semaglutide, and tirzepatide on the ACQUITY Premier Peptide CSH C18, 1.7 µm Column at three different temperatures. All three analytes and their impurities shift to earlier retention times with increasing temperature. More importantly, small changes in column temperature (±5 °C) result in selectivity differences that impact the resolution of impurity species for all three analytes.
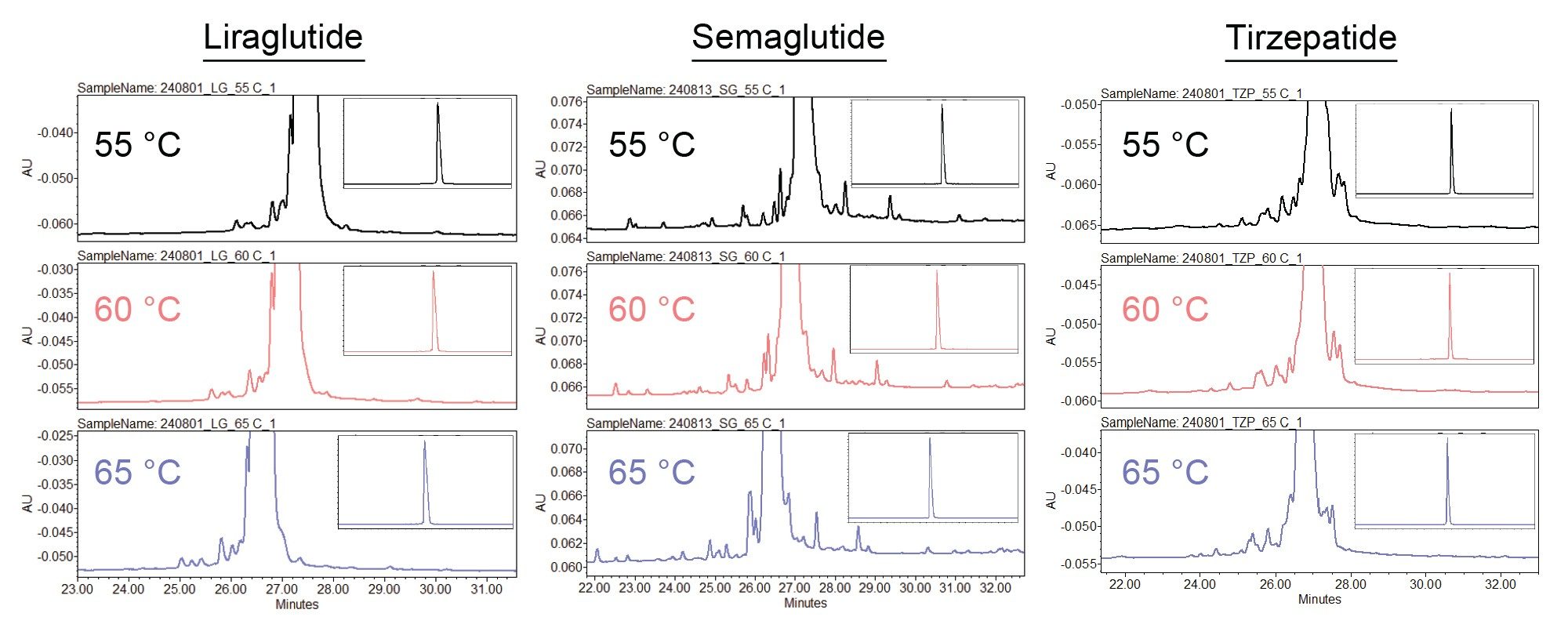
To illustrate this temperature dependent selectivity, relative retention times and peak-to-valley ratios were calculated for ten liraglutide impurity peaks. Figure 2 displays the integrated and annotated RPLC-UV results for liraglutide on the ACQUITY Premier Peptide CSH C18, 1.7 µm Column at 60 °C. Relative retention times (RRT, calculated relative to the parent peak) at each column temperature and ∆RRTs are listed in the inset table. ∆RRTs represent the difference between the maximum and minimum RRTs across the reported temperature range. All peaks except peaks 7, 8, and 10 exhibit a ∆RRT greater than 0.001. The magnitude of ∆RRT varies from peak to peak, highlighting the changes in selectivity with varying column temperature.
In Figure 3, start and end peak-to-valley (p/v) ratios for selected peaks are plotted against column temperature. The p/v ratios between peaks 2 and 3 and peaks 4 and 5 increase with increasing column temperature. In both cases, ∆RRT for the first eluting species (peaks 2 and 4) is greater than ∆RRT for the second eluting species (peaks 3 and 5), resulting in improved resolution with increasing column temperature. The p/v ratios between peaks 9 and 10 follow the opposite trend; p/v ratios decrease with increasing column temperature. The ∆RRT of peak 9 increases with increasing temperature while the RRT of peak 10 does not change, resulting in co-elution of the two species at 65 °C. Consequently, the best resolution between peaks 9 and 10 is achieved at 55 °C.
These data demonstrate the impact of column temperature on the selectivity and resolution of fatty acid modified GLP-1 RA impurity species. Changes in column temperature can improve the resolution of some impurity species while worsening the resolution of others. Column temperature can therefore be modulated to optimize resolution of critical impurity species in these separations. Importantly, the dependence of these separations on column temperature should be considered when selecting instrumentation for the reversed phase impurity analysis of fatty acid modified GLP-1 RAs. Inaccurate or poorly controlled column heating may result in variability in selectivity and resolution.4 We recommend use of an Active PreHeater (APH) (e.g. Waters p/n: 205002234) to ensure temperature equilibration of the mobile phase prior to entering the column. Further consideration should be made for laboratories that experience significant daily swings in room temperature. This can result in mobile phase temperature fluctuations that impact LC thermal control and thus method robustness.

Conclusion
The data presented here show that reversed-phase separation of liraglutide, semaglutide, and tirzepatide from their impurities is highly dependent on column temperature. Small changes in column temperature (±5 °C) result in selectivity differences that impact the resolution of impurity species for all three analytes. We anticipate similar temperature-dependence for reversed phase separation of non-GLP-1 RA lipopeptides. The temperature dependence of these separations can be used to optimize resolution of critical impurity species and the ability to deliver strict column and mobile phase temperature control should be considered when selecting instrumentation for reversed phase separation of fatty acid modified GLP1-RAs.
References
- Watanabe, J.H., Kown, J., Nan, B., Reikes, A. Trends in Glucagon-Like Peptide 1 Receptor Agonist Use, 2014 to 2022. Journal of the American Pharmacists Association. 64, 133–138. 2024.
- Clements, B.R., Rainville, P. Development of Separation Methods for GLP-1 Synthetic Peptides Utilizing a Systematic Protocol and MaxPeak™ High Performance Surface Technology. Application Note 720008267. Waters Corporation. March 2024.
- Hanna, C. M., Koza, S. M., Shiner, S. Column Selection for RPLC-UV Impurity Analysis of Fatty Acid Modified GLP-1 Receptor Agonists. Application Note 720008509. Waters Corporation. September 2024.
- Li, Z. Hong, P., McConville, P. R. The Importance of Column Compartment Thermostatting and Preheating for Temperature Sensitive Separations in Liquid Chromatography. Application Note 720007137. Waters Corporation. February 2021.
Featured Products
720008590, October 2024