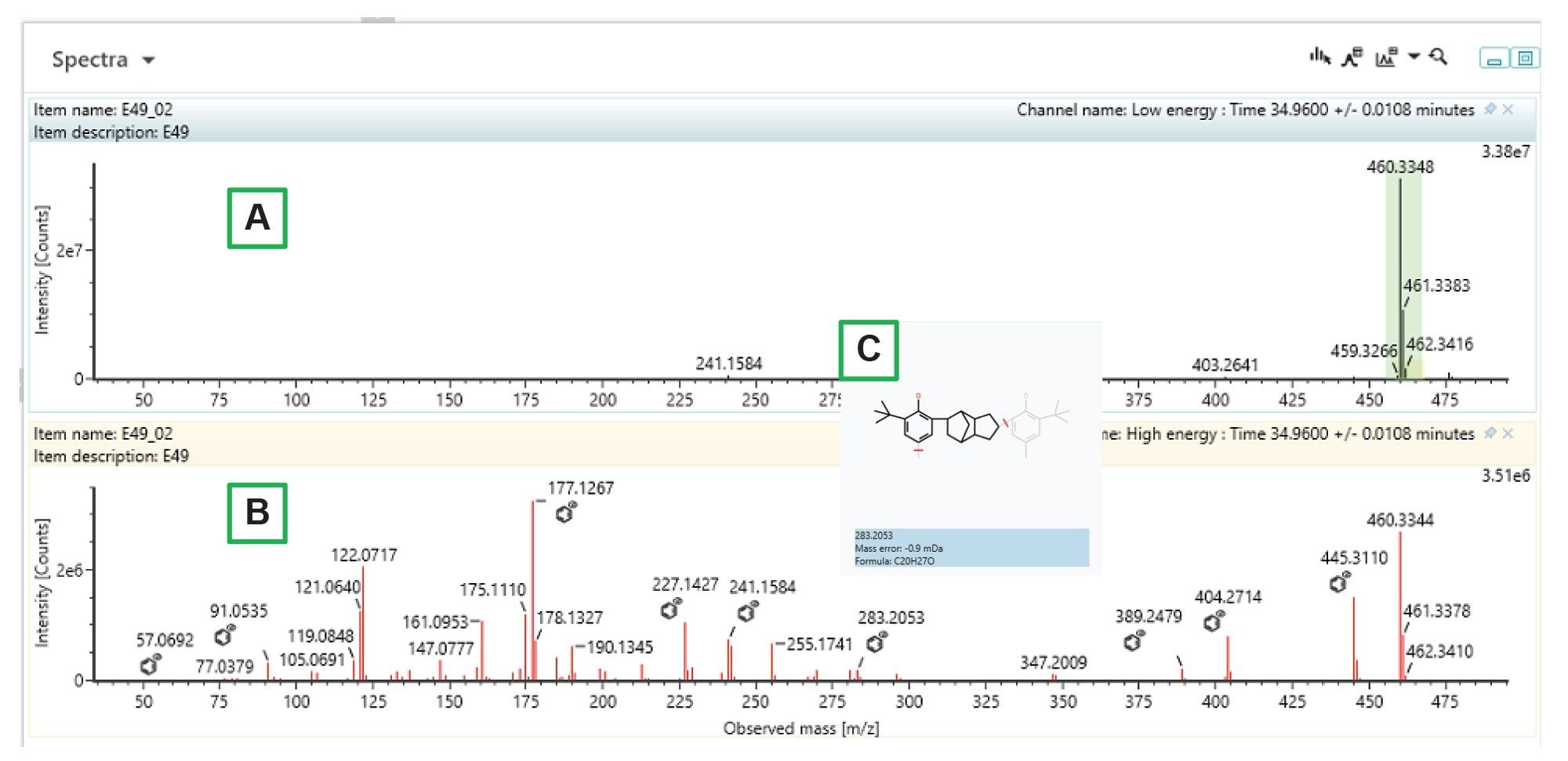
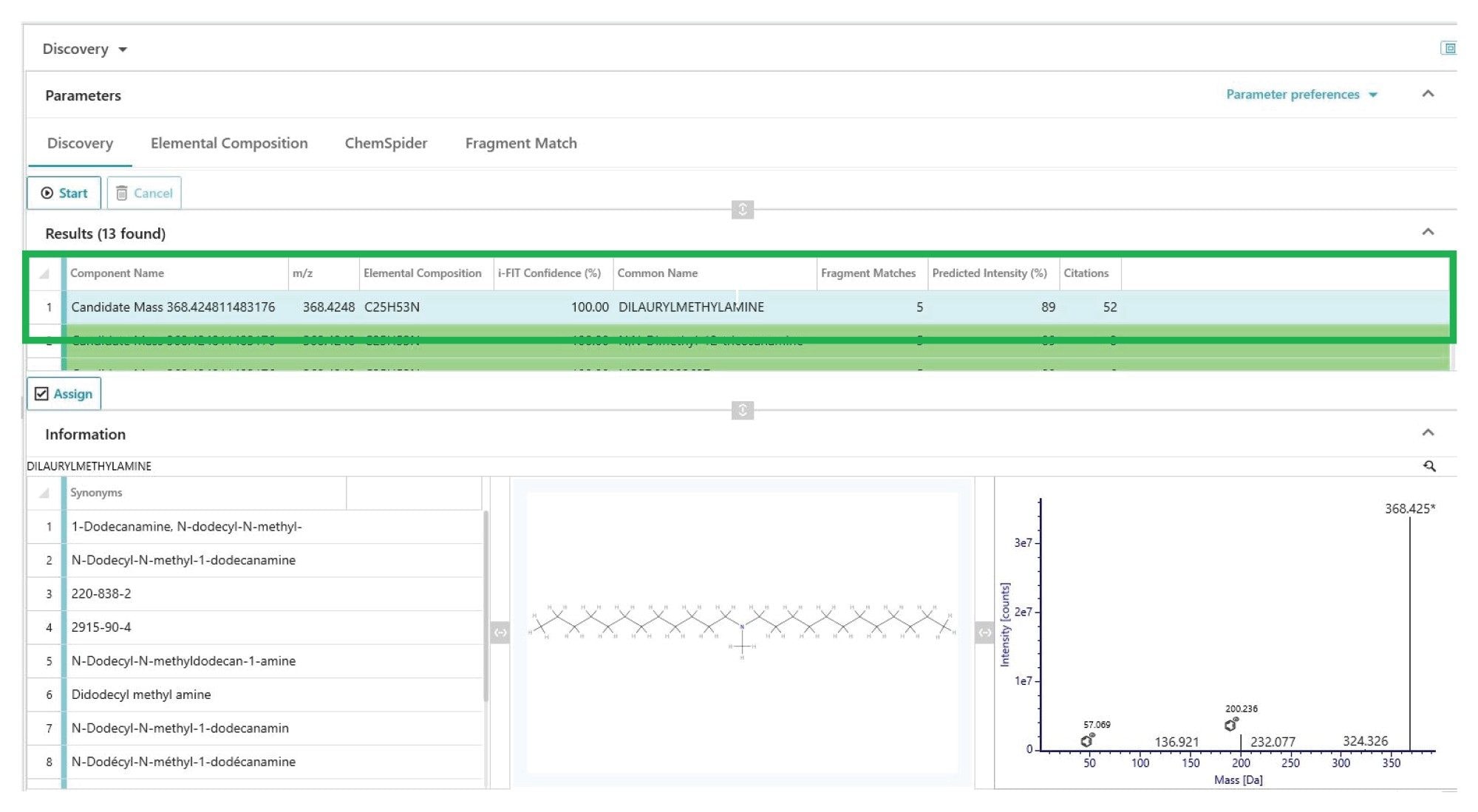
Consumer safety can be negatively impacted by chemical compounds migrating from products. Regulations are in place for industries in which the exposure routes are of higher risk, such as pharmaceuticals, medical devices, foodstuffs and cosmetics. Testing for these contaminants is commonly known as an Extractables & Leachables study.
To ensure regulatory compliance, avoid product recalls, and protect their brands, organizations must carefully control and monitor their products to eliminate the potential risks associated with extractables and leachables. Partnering with Waters means you can make critical decisions – in less time and with greater confidence by leveraging powerful technologies that are specifically designed to improve your workflow, increase testing throughput, and support compliance reporting. Our continually updated E&L library contains useful information on product ions, retention time, and collision cross section values to increase your confidence in the identification of unknowns.



Case Study: Safety Challenges of Incorporating Recycled Plastics in Food Contact Materials

Waters Xevo TQ Absolute Triple Quadrupole Mass Spectrometry delivers unprecedented levels of reliability, reproducibility, and performance for your quantification of leachables & chemicals of concern.
Maximize the sensitivity, repeatability, and robustness of your LC and LC-MS applications with the ACQUITY Premier System, featuring MaxPeak High Performance Surfaces (HPS) Technology for improvements in separation and detection to minimize the risk of undetected analytes.
Increase productivity, improve data confidence and simplify routine accurate mass workflows with the ACQUITY RDa Detector for easy access to high resolution mass measurements.
Obtain advanced optical detection perfect for routine analyses with the ACQUITY UPLC Photodiode Array (PDA) Detector that provides unprecedented trace impurity detection and quantitation with spectral analysis capabilities, from compound identification to method development.
Identify the structural elucidation of unknown compounds above the analytical evaluation threshold in less time with Waters UNIFI Application within the waters_connect platform, offering a simple workflow that includes scientific library creation, untargeted screening, multivariate statistical analysis, structural elucidation tool kit, and reporting.
Discover increases in LC efficiency, resolution, sensitivity, accuracy, speed, and reliability with ACQUITY sub-2-μm UPLC particle columns for your E&L analyses.
Designed to complement the Waters Screening Platform Solution with Waters UNIFI Application, the Extractables & Leachables Screening Standard is composed of 18 common polymer additives to evaluate and benchmark high-resolution LC-MS systems.
Achieve specified levels of cleanliness, controlled pH shifts, and adsorptive losses - from batch to batch and vial to vial using Waters Certified vials for your E&L analyses.
Analyze sample concentrations of ≤1 ng/mL effectively using TruView LCMS Certified Vials, the ideal choice for your E&L applications.
Optimize your laboratory's productivity and success with Waters Global Services. Maintain peak system performance, minimize downtime, address application challenges, and support stringent compliance requirements.
Maximize resources and minimize risk with payment options from Waters Capital, including upgrading aging equipment, getting customized support, and bundling services into one monthly payment.